Management of systemic sclerosis (SSc) is usually done through a combination of systemic and symptom-specific interventions. Standard systemic treatments focus on immunoregulation (hydroxychloroquine or intravenous immunoglobulin/IVIG) or immunosuppression (methotrexate, mycophenolate mofetil, cyclophosphamide, rituximab). Raynaud’s phenomenon and digital ulcers are almost universal in SSc and are treated with a variety of approaches, including vasodilators (calcium channel blockers, PDE5 inhibitors, prostaglandins), vasoconstrictor antagonists (endothelin-1 and angiotensin II receptor antagonists), or, in more severe cases, surgical or chemical sympathectomy. GI symptoms, such as GERD, gastroparesis, malabsorption, and small intestinal bacterial overgrowth (SIBO), are managed through a variety of mostly pharmaceutical treatments although surgical interventions are sometimes employed in severe cases. Scleroderma renal crisis (SRC) is generally treated with ACE inhibitors. To date, no medications have proven to be very effective in treating either pulmonary artery hypertension (PAH) or interstitial lung disease (ILD), and, as a result, lung-related complications from both PAH and pulmonary fibrosis (PF) are the leading causes of SSc-related mortality [1]. According to a recent study [2], it is not clear that any standard treatment for SSc has led to improved SSc survival rates over the past 40 years, beyond what would be expected by overall improvements in survival rates in the general population during this same time period.
Therapeutic Plasma Exchange
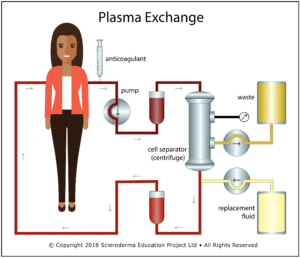
Therapeutic plasma exchange (TPE), also called therapeutic apheresis, is a procedure in which a large volume of plasma (typically 1 to 1.5 blood volumes) is replaced by a substitute fluid (most commonly 4% to 5% sterilized albumin) in a continuous flow process. Cellular components (RBC, WBC, and platelets) are separated from the plasma by either centrifugal separation or filtration, combined with the replacement fluid, and returned in a process that typically takes 1.5 to 2 hours. In the US, almost all TPE is done using centrifugal separation. A related procedure – plasmapheresis – removes a smaller amount of plasma (typically less than 15% of blood volume) that is inadequate to cause significant hypovolemia, so no replacement fluid is required. Unfortunately, the terms “therapeutic plasma exchange” and “plasmapheresis” are often used interchangeably in the published literature, creating potential confusion when researching the effects of TPE.
The usual rationale and the primary post hoc explanation for any benefits seen from TPE is that TPE treatments temporarily reduce the levels of circulating factor(s) (e.g., autoantibodies or immune complexes, cytokines or adhesion molecules) that are presumed to be involved in SSc disease pathogenesis. A single TPE treatment of 1 to 1.5 blood volumes removes approximately 65% of any potential circulating pathogenic factors [3]. It is important to note that certain plasma components are also present in the extravascular space so post-TPE plasma concentrations may be different than expected, due to tissue-plasma equilibration [4].
TPE has been tried as a possible treatment for SSc since 1978. While TPE is rarely used as a treatment modality for SSc in the US, it is more commonly used in Europe and is a mainline, government-approved treatment option in Italy [5]. Medicare and some US healthcare companies cover TPE as an available treatment option for SSc patients who are unresponsive to conventional therapy [6]. The American Society for Apheresis currently classifies TPE for treating systemic sclerosis treatment as a Category III treatment: “Optimum role of apheresis therapy is not established. Decision making should be individualized.” [7].
Efficacy of TPE as a Treatment for SSc
A recent comprehensive review of the use of TPE as a treatment for SSc [8] looked at 46 published papers involving a total of 572 patients. Nineteen of the articles were case reports, involving a total of 26 patients. The remaining 27 articles (546 patients) ranged from letters to the editor describing a small group of patients treated with TPE to a large-scale review of 102 patients treated over a 15-year period at a single clinic in Italy. Out of the 572 patients, 455 received TPE. The rest were in control groups. To the extent that it could be determined, about 75% of the patients were diagnosed with diffuse cutaneous systemic sclerosis (dcSSc), 23% were diagnosed with limited cutaneous systemic sclerosis (lcSSc), and 2% had mixed connective tissue disease (MCTD).
The major conclusions of this review were:
- In almost all studies, the majority of patients receiving TPE showed improvements in both symptoms and laboratory markers, whether in short-term treatment of crisis situations or from long-term administration of regular TPE.
- Many patients experienced significant improvement in Raynaud’s symptoms and demonstrated initial healing of digital ulceration after just three to four weekly treatments.
- While the effects of even a few TPE treatments often lasted for several months, only continued long-term treatments resulted in stabilization of symptoms or, in one recent case report, sustained remission over a 23-year period.
- Venous access problems occurred in a minority of patients receiving long-term TPE, leading to cessation of TPE treatments in some cases and switching to central venous access in other cases.
- TPE was very well tolerated by almost all patients. Adverse events were rare and, in almost all cases, mild with no reported deaths.
Effects of TPE on Raynaud’s Phenomenon (RP) and Digital Ulcers (DU)
Treatment of RP and DU in SSc is challenging and in some cases inadequate to prevent progression to gangrene and eventual digit amputation. One of the more surprising findings in 16 of the papers reviewed [8] was the fact that three or four TPE treatments weekly often led to complete cessation of Raynaud’s attacks and healing of even long-standing DU. These effects were long-lasting, with RP not returning for six months or longer, and in one study [9], patients had no return of DU during a three-year follow up.
Standard treatments for RP and DU in SSc are focused on improving distal blood flow by either increasing vascular dilation or reducing vasoconstriction or vasospasm. Since TPE treatments are not known to directly increase vasodilation or reduce vasoconstriction or vasospastic activity, these results raise the possibility that an entirely different mechanism of action may be involved in the observed improvements in RP and DU healing following TPE.
Effects of Long-Term TPE
Only a small number of studies have examined the efficacy of long-term TPE on patients with SSc. A 2001 Italian study [10] compared pre- and post-TPE laboratory markers reflecting disease activity in a group of 28 Italian patients who received regular TPE combined with D-penicillamine over a six-year period (mean 33 months) against a control group of 25 SSc patients who received D-penicillamine alone. Significant improvements in clinical scores and laboratory markers only occurred in the TPE treatment group even though at pre-treatment the TPE group had worse laboratory measures and clinical scores than the control group.
A second Italian study [11] summarized the results of long-term treatment of 97 SSc patients using TPE as an adjunct treatment in addition to D-penicillamine or an immunosuppressant. While the authors rated TPE efficacy as either “excellent” or “good” in 52.4% of the patients, the simultaneous use of adjunct treatments make it impossible to determine to what extent these positive effects are attributable to TPE.
Szekanecz [12] followed a male patient with dcSSc for 11 years. The patient received a combination of regular TPE treatments combined with IVIG during the first year and was maintained on a reduced frequency of TPE/IVIG during the 10-year follow up period. Unfortunately, because of the simultaneous use of TPE and IVIG, it is impossible to determine if the observed improvements were from TPE, IVIG, or a synergistic combination of both.
Hertzman [13] treated a 12-year-old patient diagnosed with Mixed Connective Tissue Disease (MCTD) with an initial series of ten TPE treatments over a five and one-half week period, resulting in significant improvement in nodular lesions and complete elimination of hand swelling. TPE was reduced to one TPE every three weeks and the patient remained asymptomatic at two-year follow-up with no other treatment intervention.
A 2017 very long-term (22 year) case report [14] documented the effects of regular TPE as the sole systemic intervention in a patient with rapidly progressing anti-centromere positive limited SSc. Pre-treatment the patient had severe GERD, Raynaud’s, and reduced DLCO/VA (diffusing lung capacity for carbon monoxide corrected for alveolar volume). TPE was administered in a pulsed protocol (one TPE per week for four weeks, eight weeks no TPE, repeat). All symptoms (except for very mild residual Raynaud’s), including reduced DLCO/VA, disappeared after two to three years. The patient remains in excellent health with continued regular TPE treatments on the original pulsed protocol (approximately 370 to date); however, dropping or reducing TPE treatment frequency led to an eventual return of GI symptoms in two attempts to alter the treatment protocol.
TPE Complications
Eleven of the 46 papers reviewed [8] described complications directly related to the use of TPE. There were two main types of complications: 1) venous access issues, and 2) short-term side effects directly associated with the TPE procedure. There were no reported fatalities associated with TPE and short-term side effects were generally minor and usually did not prevent TPE from being completed. In one early study [11] four patients (out of 40) had allergic reactions. This primarily occured only when fresh frozen plasma was used instead of sterilized albumin. In a small percentage of the cases, venous access difficulties prevented TPE from being performed using the preferred method of peripheral venous access, leading to cessation of TPE. In other cases, implanted central venous catheters were used for short-term TPE or an arteriovenous fistula was surgically created for long-term TPE.
When Does TPE Fail to Work in Patients with SSc?
Guillevin et al. [15] tried TPE treatments in seven patients with severe diffuse SSc after failure of other treatments. Disease duration at time of initial TPE averaged eight years. In three patients, TPE treatments had to be stopped because of venous access problems. In the remaining four patients, only one showed benefit: improvement of articular and cutaneous symptoms. This suggests that TPE may not be effective in late stages of dcSSc.
Capodicasa et al. [16] tried TPE in two patients in scleroderma renal crisis (SRC). While brief improvement was seen in one patient, the authors concluded that TPE would need to be started earlier to be potentially effective. In contrast to all other reports reviewed in this paper, this study used membrane TPE instead of centrifugal TPE. Also, ACE inhibitors are now employed as the treatment of choice for treating SRC.
Kfoury et al. [17] tried intensive TPE on an 85-year-old patient admitted because of SRC with the rare complication of thrombotic thrombocytopenic purpura. Intense TPE starting with one week of daily TPE treatments increasing to twice a day for an additional week had no effect, and the patient died shortly after cessation of TPE and all medications secondary to pulmonary and cardiac conditions related to SRC.
While TPE was not effective in all patients in studies with overall positive outcomes, few data were presented about patients who failed to respond to TPE treatments. Nevertheless, most authors clearly felt that TPE would be most effective if started early in the disease process.
TPE and Mixed Connective Tissue Disease
No clinical trial or other large-scale study of TPE as a potential treatment for MCTD has been done to date. The TPE treatment paper [8] examined six MCTD case reports. Most of the case reports were focused on the use of short-term TPE to deal with an acute issue, such a renal failure or central retinal vein occlusion. However, one paper [18] followed a 12-year-old MCTD patient who went into remission after five and a half weeks of TPE (ten treatments total) and remained in remission with regular maintenance TPE at the two-year follow-up. While MCTD has overlapping symptoms of SLE, it is interesting to note that TPE was not effective in patients with systemic lupus erythematosus (SLE) in a short-term randomized controlled trial [19].
Why Does TPE Show Positive Results?
Reduction of Potential Circulating Pathogenic Factors
Many antibody-mediated diseases are due to IgG antibodies (~150 kDa). Blood plasma and extravascular extracellular fluid within the body contain about 45% and 55% of total IgG, respectively [20]. Thus, the single blood volume TPE treatment could theoretically remove ~30% of circulating IgG. Due to extravascular to intravascular circulation during a TPE treatment, the actual removed amounts of IgG are somewhat higher than expected [21]. Nevertheless, within two days, plasma IgG levels return to about 70% of pre-TPE levels [22].
The long-lasting effects of TPE in SSc patients suggest that the mechanism of action may be independent of the reduction of circulating antibodies. Specifically, several studies have documented six-month (or longer) beneficial effects following a single series of four TPE/week treatments. These favorable effects on both laboratory markers and clinical symptoms cannot be easily explained by short-lived reductions in circulating antibodies [9,23,24]. Also, when comparing the effects of standard plasma exchange (PE) with “placebo plasma exchange (PPE),” where patient’s cellular blood elements were re-mixed with the patient’s own separated plasma (instead of replacing the plasma with 4% to 5% sterilized albumin), McCune et al. [25] noted that, “There appears to be no difference between plasma and placebo exchange as measured in the vascular laboratory.”
Is Blood Rheology the Key?
Over the past 42 years, many published papers have documented that blood rheology is abnormal in patients with SSc. Individual papers have commented on or measured differing aspects of this abnormal rheology, including elevated whole blood viscosity (WBV), increased plasma viscosity (PV), decreased RBC deformability, and abnormal red blood cell (RBC) aggregation. This topic is explored in detail in the Research / SSc and Abnormal Blood Rheology section of this website:
Issues/Concerns About the Use of TPE for Treating SSc
Safety and Complications
While TPE is generally not used for treating SSc currently (at least in the US), it is a widely-used procedure for many other autoimmune disorders, for example myasthenia gravis, Guillain-Barré, chronic demyelinating polyneuropathy, and Goodpasture’s syndrome. This broad usage of TPE prompted several large-scale studies to assess TPE safety and complication rates.
Cid et al. [26] reviewed the efficacy and safety of TPE in 317 patients and 2730 procedures over an 11-year period. Observed adverse events occurred in only 3% of procedures. In all cases the adverse events were mild and transient, and patients were able to complete the scheduled TPE treatment. Similarly, in a study of more than 20,000 therapeutic apheresis procedures performed in Sweden [27], mild adverse events requiring no intervention occurred 1.5% of the time, moderate events not requiring cessation of treatment occurred 2.8% of the time, and severe events requiring cessation of treatment occurred 0.8% of the time. There were no fatalities.
The most severe complications in TPE occur with fresh frozen plasma as the replacement fluid. Almost all studies of TPE for treating SSc used sterilized 5% albumin, which has a much better safety profile because of substantially reduced risk of anaphylactic type events.
The most common short-term problem with TPE is hypocalcemia, usually presenting as mild paresthesias or perioral tingling from the use of citrate as an anticoagulant. Prophylactic use of oral calcium supplements is usually adequate to prevent or minimize TPE-associated hypocalcemia. Some patients may experience mild hypotension, muscle cramps, or mild headaches from hypovolemia especially with lower concentrations of albumin than the recommended 5% solution.
Vascular Access
The safest way to perform TPE is using regular peripheral venous access. Venous access problems were discussed in several of the reviewed articles and were often the reason for discontinuation of TPE. While the exact percentage of patients who would require alternatives to peripheral venous access for long-term TPE is not clear, the data indicate that most patients can undergo long-term TPE using normal peripheral access. Khatri et al. [28], summarizing the results from more than 60,000 TPE treatments, indicates that peripheral venous access is successful in about 75% of the procedures performed at their clinic. However, two new venous access techniques are now available that should increase the likelihood of long-term peripheral venous access: 1) vein illumination technology such as VeinViewer™ and AccuVein™, and 2) ultrasonic-guided peripheral venous cannulation [29].
For patients who cannot undergo normal peripheral venous access, there are a number of alternatives that are available. Central catheters are not a good option for most patients for long-term TPE because of the significant infection risk. Alternatives such as surgically created fistulas or implantable vascular-access devices (ports), such as PowerPorts™ or Vortex™, may be better options for very-long term use of TPE if peripheral venous access is not an option.
Cost
Winters [30] did an analysis of TPE cost and determined that each treatment cost a little under $1,200 when TPE was performed using albumin. Average Medicare reimbursement rates (2015) are about $1,140 plus the cost of albumin, which varies depending on the size of the patient. Several studies suggest that between 12 and 18 treatments per year may be sufficient to control SSc symptoms. For instance, the 16 TPE treatment/year protocol discussed in Harris [14] translates into an annual cost of about $20,000 per year.
A recent study of the annual cost of modern biologic drugs now commonly used to treat rheumatoid arthritis and other autoimmune conditions [31] indicated that the lowest price biologic – Humira (adalimumab) – was about $21,000 per year. Other biologics were somewhat higher. This suggests that annual costs for long-term TPE, while significant, are similar to standard pharmacological options used for other autoimmune diseases.
IVIG, which is being increasingly tried as a treatment for SSc [32,33], is much more expensive than TPE. A typical treatment regimen in these early studies used a dosing of 2 gm/kg monthly. Using data from Winters [34], this works out to more than $10,000 per month for a typical 70-kg patient, i.e., approximately $120,000/yr.
Summary and Conclusion
The preponderance of evidence suggests that long-term TPE may offer a low-risk and cost-effective way to control, and in some cases, reverse SSc symptoms and signs. In contrast to current immunosuppressive treatments that carry significant risk, long-term TPE appears to be safe, well–tolerated, and associated with only very few, mostly minor side effects. While TPE is not an inexpensive procedure, annual costs are similar to modern pharmaceuticals commonly used to treat SSc and other autoimmune diseases.
The current American Society for Apheresis Guidelines suggest that clinicians should make individual decisions on the suitability of TPE as a treatment for their patients with SSc. If clinicians do decide to try TPE on an individual basis, it is important that they also try to extract as much useful research data as possible from any such individual trials. We have prepared several documents that may be a useful starting point for clinicians who are considering trying TPE. These documents are listed below in the Resources section of this article.
References
- Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66(7):940-944. doi:10.1136/ard.2006.066068.
- Elhai M, Meune C, Avouac J, Kahan A, Allanore Y. Trends in mortality in patients with systemic sclerosis over 40 years: a systematic review and meta-analysis of cohort studies. Rheumatology (Oxford). 2012;51(6):1017-1026. doi:10.1093/rheumatology/ker269.
- Patten E, Berkman EM. Therapeutic Plasmapheresis and Plasma Exchange. CRC Crit Rev Clin Lab Sci. 1986;23(2):147-175. doi:10.3109/10408368609165798.
- Winters JL. Plasma exchange: concepts, mechanisms, and an overview of the American Society for Apheresis guidelines. Hematol Am Soc Hematol Educ Progr. 2012;2012:7-12. doi:10.1182/asheducation-2012.1.7.
- MINISTERIAL DECREE May 28, 1999, no. 329. Regulation laying down rules for the detection of chronic and disabling diseases within the meaning of Article 5 (1) (a) of Legislative Decree no. 124. (GU General Series No.226 of 25-9-1999 – Ordinary Supplement No 174).
- National Coverage Determination (NCD) for Apheresis (Therapeutic Pheresis) (110.14).
- Schwartz J, Padmanabhan A, Aqui N, et al. Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach from the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher. 2016;31(3):149-338. doi:10.1002/jca.21470.
- Harris ES, Meiselman HJ, Moriarty PM, Metzger A, Malkovsky M. Therapeutic plasma exchange for the treatment of systemic scleroderma: a comprehensive review and analysis. Manuscript currently in review. Contact the author for a copy.
- Jacobs MJ, Jörning PJ, Van Rhede van der Kloot EJ, et al. Plasmapheresis in Raynaud’s phenomenon in systemic sclerosis: a microcirculatory study. Int J Microcirc Clin Exp. 1991;10(1):1-11.
- Cozzi F, Marson P, Rosada M, et al. Long-term therapy with plasma exchange in systemic sclerosis: effects on laboratory markers reflecting disease activity. Transfus Apher Sci. 2001;25(1):25-31.
- Marson P, Cozzi F, Silvestro G De. Il trattamento a lungo termine con plasma-exchange nella sclerosi sistemica. La Trasfus Del Sangue. 2001;46.
- Szekanecz Z, Aleksza M, Antal-Szalmás P, et al. Combined plasmapheresis and high-dose intravenous immunoglobulin treatment in systemic sclerosis for 12 months: follow-up of immunopathological and clinical effects. Clin Rheumatol. 2009;28(3):347-350. doi:10.1007/s10067-008-1062-2.
- Hertzman, Alex; Cooke, Charles L.; Rodriquez,Gilberto E.;.Sharp D. Treatment of childhood mixed connective tissue disease with plasmapheresis. Clin Immunol Newsl. 1981;2(18):142-144. doi:10.1016/S0197-1859(81)80066-0.
- Harris E, Meiselman H, Moriarty P, Weiss J. Successful long-term (22 Year) treatment of limited scleroderma using therapeutic plasma exchange: Is blood rheology the key? Clin Hemorheol Microcirc. 2017;65:131-136. doi:10.3233/CH-16140.
- Guillevin L, Leon A, Levy Y, et al. Treatment of progressive systemic sclerosis with plasma exchange. Seven cases. Int J Artif Organs. 1983;6(6):315-318.
- Capodicasa G, De Santo NG, Galione A, et al. Clinical effectiveness of apheresis in the treatment of progressive systemic sclerosis. Int J Artif Organs. 1983;6 Suppl 1:81-86.
- Kfoury Baz EM, Mahfouz RA, Masri AF, Jamaleddine GW. Thrombotic thrombocytopenic purpura in a case of scleroderma renal crisis treated with twice-daily therapeutic plasma exchange. Ren Fail. 2001;23(5):737-742.
- Hertzman, Alex; Cooke, Charles L.; Rodriquez,Gilberto E.;.Sharp D. Treatment of childhood mixed connective tissue disease with plasmapheresis. Clin Immunol Newsl. 1981;2(18):142-144. doi:10.1016/S0197-1859(81)80066-0.
- Wei N, Klippel JH, Huston DP, et al. Randomised trial of plasma exchange in mild systemic lupus erythematosus. Lancet (London, England). 1983;1(8314-5):17-22.
- Raimann, J. G. (2015), Handbook of Dialysis Fifth Edition by John T. Daugirdas, Peter G. Blake and Todd S. Ing. Philadelphia, PA: Lippincott Williams & Wilkins, 2014, 900 pp. (ISBN-13: 978-1451144291). Hemodial Int, 19: 609–610. doi:10.1111/hdi.12322
- Derksen RH, Schuurman HJ, Meyling FH, Struyvenberg A, Kater L. The efficacy of plasma exchange in the removal of plasma components. J Lab Clin Med. 1984;104(3):346-354.
- Brecher ME. Plasma exchange: why we do what we do. J Clin Apher. 2002;17(4):207-211. doi:10.1002/jca.10041.
- Hamilton W, White J, Cotton L. Circulatory improvement in Raynaud’s phenomenon following plasma exchange. In: Sieberth HG (Ed) Plasma Exchange. Stuttgart New York: Schattauer; 1980:301-307.
- Ding C, Zhang X. [A prospective study of plasma exchange in the treatment of diffuse scleroderma]. Zhonghua nei ke za zhi. 1995;34(9):616-619.
- McCune MA, Winkelmann RK, Osmundson PJ, Pineda AA. Plasma exchange: a controlled study of the effect in patients with Raynaud’s phenomenon and scleroderma. J Clin Apher. 1983;1(4):206-214.
- Cid J, Carbassé G, Andreu B, Baltanás A, Garcia-Carulla A, Lozano M. Efficacy and safety of plasma exchange: ann 11-year single-center experience of 2730 procedures in 317 patients. Transfus Apher Sci. 2014;51(2):209-214. doi:10.1016/j.transci.2014.08.018.
- Mokrzycki MH, Balogun RA. Therapeutic Apheresis: A Review of Complications and Recommendations for Prevention and Management. J Clin Apher. 2011;26(5):243-248. doi:10.1002/jca.20303.
- Khatri B, Kramer J. Vascular access for therapeutic plasma exchange. Muscle Nerve. 2013;48(4):624. doi:10.1002/mus.23873.
- Costantino TG, Parikh AK, Satz WA, Fojtik JP. Ultrasonography-guided peripheral intravenous access versus traditional approaches in patients with difficult intravenous access. Ann Emerg Med. 2005;46(5):456-461. doi:10.1016/j.annemergmed.2004.12.026.
- Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C. Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barré syndrome. BMC Health Serv Res. 2011;11:101. doi:10.1186/1472-6963-11-101.
- Howe A, Eyck L Ten, Dufour R, Shah N, Harrison DJ. Treatment patterns and annual drug costs of biologic therapies across indications from the Humana commercial database. J Manag care Spec Pharm. 2014;20(12):1236-1244.
- Cantarini L, Rigante D, Vitale A, et al. Intravenous immunoglobulins (IVIG) in systemic sclerosis: a challenging yet promising future. Immunol Res. 2015;61(3):326-337. doi:10.1007/s12026-014-8615-z.
- Poelman CL, Hummers LK, Wigley FM, Anderson C, Boin F, Shah AA. Intravenous Immunoglobulin May Be an Effective Therapy for Refractory, Active Diffuse Cutaneous Systemic Sclerosis. J Rheumatol. 2015;42(2).
- Winters JL, Brown D, Hazard E, Chainani A, Andrzejewski C. Cost-minimization analysis of the direct costs of TPE and IVIg in the treatment of Guillain-Barré syndrome. BMC Health Serv Res. 2011;11:101. doi:10.1186/1472-6963-11-101.
