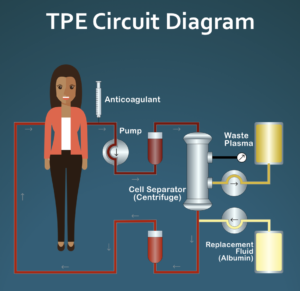
Therapeutic plasma exchange (TPE/therapeutic apheresis) is a process in which most of the plasma (the liquid part of blood) is replaced by a substitute fluid such as sterilized albumin in an outpatient procedure that typically takes 1.5 to 2 hours. The goal of TPE is to remove large amounts of potential disease causing proteins, for example autoantibodies, that mistakenly attack our own bodies. TPE is a widely used procedure for a variety of neurological diseases as well as some blood disorders, but is rarely used in the US as a treatment for systemic sclerosis (SSc). It is used more often in Europe and is a government approved treatment option for SSc in Italy.
A recent comprehensive review of the research literature on the use of TPE as a treatment for patients with systemic scleroderma (Link to TPE Review) concluded that:
“The preponderance of evidence suggests that long-term TPE may offer a low-risk and cost-effective way to control,and in some cases reverse,SSc symptoms and signs. In contrast to current immunosuppressive treatments that carry significant risk, long-term TPE appears to be safe, well–tolerated, and associated with only very few, mostly minor side effects. While TPE is not an inexpensive procedure, annual costs are similar to modern pharmaceuticals commonly used to treat SSc and other autoimmune diseases.”
When preparing an earlier version of this review paper for presentation at a medical conference in 2016, we conducted an informal survey of several major US insurance companies to determine whether or not they would cover TPE treatments for patients with diagnosed SSc. What we found then was that all insurance companies that we contacted considered TPE to be experimental/investigational, and would either not be covered at all or only under very limited circumstances. The one exception was Medicare which indicated that TPE is covered for scleroderma if the disease is life threatening other treatments are not working. At that time, we were aware of only one patient with Medicare who was receiving TPE in the US on a routine basis (Link to Case Report). When the patient turned 65 and switched from private insurance to Medicare, it was covered immediately with no initial rejection, suggesting that Medicare would likely cover TPE for other patients as well.
In preparation of a new paper that we are writing on the use of TPE to treat Raynaud’s and digital ulcers in SSc patients who are not responding to conventional treatments, we again conducted an informal survey to see if the availability of insurance coverage for TPE had changed since our previous survey in early 2016. In addition to our own review of published information on TPE coverage on major insurance company websites, we asked SSc patients to individually contact their insurance companies and ask if they covered TPE (CPT code: 36514) for a diagnosis of diffuse systemic sclerosis (ICD10 code: M34.0) or limited systemic sclerosis (M34.1). Patients were asked to enter the information from their insurance companies into an anonymous online survey.
The results of this recent survey, combined with our own independent review of national plans, suggests that insurance coverage for TPE as a treatment option for patients with diagnosed SSc is now widely available in the US. Some plans require pre-authorization, and in all cases, coverage is subject to normal deductibles and co-pays. In the case of Aetna, for example, their current guidelines (Aetna coverage for TPE) indicate that TPE is covered for “scleroderma and polymyositis, in persons who are unresponsive to conventional therapy.” Basically, if the doctor indicates that TPE is medically necessary, 100% of the plans surveyed indicated that they will now cover it. This does not guarantee that all plans will cover TPE, but the results of our survey suggest that TPE is likely to be available as a covered treatment option for many SSc patients.
While TPE has a very good safety profile with no known long-term risks or side effects, it may or may not be a suitable treatment option for individual patients for a variety of reasons. These may include, but are not limited to, the stage of the disease, local availability of equipment and trained personnel, and potential venous access issues. Patients who are interested in learning more about TPE may find it helpful to start by viewing this educational video for clinicians: https://youtu.be/iWT0oW8FRdE. (Note that this video has not been revised to reflect our updated information on insurance coverage in the US.) We also recently published a blog post that gives additional information on TPE that is not in the video: TPE: Additional Q & A.
With insurance coverage for TPE now likely to be available to many SSc patients in the United States, the main barrier in discussing this treatment option with your doctor is likely to stem from the fact that some rheumatologists may not be very familiar with TPE. While TPE is a common treatment for many neurological and hematological diseases, it is rarely used as a routine treatment for rheumatic diseases. We have written an article that may be helpful if you are interested in discussing TPE with your doctors: http://sclerodermainfo.org/pdf/TPE-Talking.pdf.

Most rheumatologists are just learning about this treatment and haven’t read the research, so getting someone to order it can be challenging. This blog post is a good place to start: http://sclerodermainfo.org/pdf/TPE-Talking.pdf. Also, watch this video: https://youtu.be/iWT0oW8FRdE.