 We are frequently asked by patients interested in talking to their doctor about trying therapeutic plasma exchange (TPE) how to approach this topic. This can be very challenging, especially since many rheumatologists have little or no experience using TPE and often view TPE as a last resort treatment when everything else fails, assuming (incorrectly) that it is very invasive and risky.
We are frequently asked by patients interested in talking to their doctor about trying therapeutic plasma exchange (TPE) how to approach this topic. This can be very challenging, especially since many rheumatologists have little or no experience using TPE and often view TPE as a last resort treatment when everything else fails, assuming (incorrectly) that it is very invasive and risky.
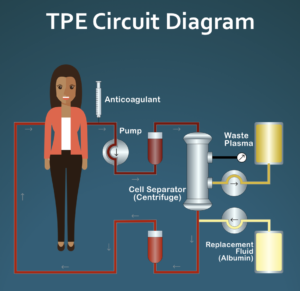
Working with the doctors on our Medical Advisory Board, we have written a document that gives clinicians a quick overview of this experimental treatment option. The document gives basic information about therapeutic plasma exchange, discusses the potential role of abnormal blood rheology in systemic sclerosis pathogenesis, and describes a specific pulsed plasma exchange (PPE) protocol that is focused on improving blood rheology instead of the more typical uses of TPE, which are to reduce levels of plasma circulating pathogenic molecules such as autoantibodies. The document briefly discusses safety, cost, and venous access issues that will be of concern to the clinician if s/he is considering this experimental treatment approach. The document also includes links to additional resources for clinicians and patients, including details of the suggested pulsed plasma exchange protocol and an introductory article about TPE for patients.
Links to this document in both US and A4 formats are included in the Additional Articles for Clinicians section of this website. Here are direct links to the article in both US and A4 formats:
- US Format: https://sclerodermainfo.org/pdf/PPE-Overview-US.pdf
- A4 Format: https://sclerodermainfo.org/pdf/PPE-Overview-A4.pdf
Before initiating a discussion with your doctor on this treatment option, it may make sense to first see if your insurance covers therapeutic plasma exchange. In the US, most plans do, but not all. To determine if your insurance covers TPE, call their customer service number and ask if they cover therapeutic apheresis, CPTD code 36514 for the treatment of systemic sclerosis, ICD 10 code M34.0 (if diffuse) or M34.1 (if limited/CREST). Some plans will require pre-authorization if they do cover it, meaning that your doctor would have to submit a letter indicating that this treatment is necessary for you.

To date, nobody has been able to try this in Canada or the UK. I don’t have information about other countries but it should be possible in Italy.
any information about treatment in Canada or other countries