 Last week, we had a Zoom call that included eight of the nine people who are now individually trying an experimental treatment called pulsed plasma exchange (PPE) to treat their systemic sclerosis (SSc). Seven of the patients are in the US and one was in Moscow. The missing person lives in Turkey. The call was very interesting so I thought I would summarize some of the key points that came up during the two hour call.
Last week, we had a Zoom call that included eight of the nine people who are now individually trying an experimental treatment called pulsed plasma exchange (PPE) to treat their systemic sclerosis (SSc). Seven of the patients are in the US and one was in Moscow. The missing person lives in Turkey. The call was very interesting so I thought I would summarize some of the key points that came up during the two hour call.
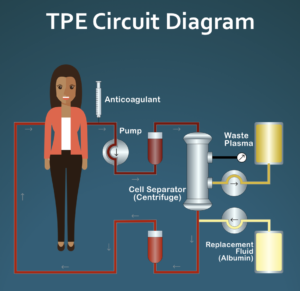
For those of you who are not familiar with PPE, this is an “off-label” use of a common treatment (therapeutic plasma exchange/TPE). The protocol is pulsed, meaning that it starts and stops. The actual protocol is very simple: one TPE treatment per week for four weeks followed by eight weeks of rest, repeat indefinitely. While TPE is commonly used to treat a number of different hematological and neurological conditions, often as an intense treatment for an acute condition, e.g., Guillain-Barré syndrome, is rarely used to treat rheumatological conditions such as SSc. TPE has an excellent safety profile with only one known long-term risk/side effect that is discussed below.
The American Society for Apheresis (ASFA) formally publishes evidence based guidelines on the use of TPE for various conditions. The current edition of the Guidelines classifies TPE as a treatment for systemic sclerosis as a Category III treatment. Basically, a Category III treatment is one where there are no studies suggesting that TPE is ineffective, but there are also not enough very well done studies, e.g., randomized controlled trials, to formally evaluate how effective it is for the specific condition. Because of this, clinicians should make their own individual decisions on trying TPE. As there are currently no formal clinical trials underway trying TPE for treating, all of the nine patients are trying PPE on an individual basis. However, since all of the patients are using the exact same protocol, this should allow a lot of data to be collected on these individual trials that may help to advance the research on this experimental treatment option.
(Full disclosure: I am an SSc patient myself, formally diagnosed in January 1990. You can read my backstory here if interested: http://sclerodermainfo.org/pdf/Ed.pdf. What is relevant here is that I am the person who invented this protocol back in 1993 and I have been on it since November 1993. I went into full clinical remission by 1996 with complete reversal of all symptoms, including early lung damage (over a longer period of time), with the exception of very minor residual Raynaud’s. Symptoms such as severe GERD gradually disappeared over a two year period and have never returned as long as I stay on the protocol (more on this in a bit). Over the years I became a scleroderma educator and starting about six years ago, started focusing on research on this rare disease. For the past several years I have had a formal appointment as an honorary fellow in rheumatology at the Univ. of Wisconsin in Madison. I have several publications in the field including my own case report and a comprehensive review of 46 published studies on the use of TPE as an experimental treatment for SSc.)
About three years ago, a second patient (located in Europe) started on my PPE protocol to deal with her early stage diffuse scleroderma (with a rare U3-RNP antibody). She went into full remission after about nine months. A little more than a year ago, the first new US patient started on the protocol quickly followed by a second patient in the US. Since then, five additional patients have started on the protocol, in some cases, very recently. Five of the patients are positive for centromere antibodies (including me). One has Scl-70 antibodies, another U3-RNP antibodies, and one is ANA positive but is negative for all 10 of the known scleroderma specific antibodies but has a formal diagnosis of diffuse systemic sclerosis based on her clinical profile.
Since I am now starting to work on an initial case series for publication that will include three or four of the longer term patients, I thought it might be useful to see if the patients were interested in a Zoom session to share their own experiences to date, either good or bad, raise issues and concerns, and see if they could learn anything that would be helpful in their own individual case as well as help in preparing the case series.
I won’t go into a lot of detail here, but here are some of the highlights of the call:
- Being educated about what to expect when starting PPE was very helpful. Several years ago I co-authored an article with the head infusion center nurse at the Univ. of Wisconsin Hospital titled “TPE for Newbies”. All patients found this very helpful in knowing what to expect. I wish I had had a copy of this back in 1993 when I first started and had no idea what getting TPE would be like. 🙂
- All of the patients who had been on the protocol for about a year or longer had significant benefit to date. In addition to the second patient (who was unable to make the call) mentioned above, the third patient was seeing clear improvement in one of her major GI symptoms (GAVE), which is basically unheard of in the research literature. The fourth patient on the protocol has gradually experienced major improvements in her GERD during this time period. Interestingly, her experience closely duplicates my own experience one the protocol in 1993-1994. She also has centromere antibodies. The other patients started PPE much more recently. All but one indicated at least some apparent improvements, but I think it is too early to know for sure how PPE will work in this newer group of patients. One patient had not had any improvement so far. She has diffuse disease with significant skin issues and this is a symptom where there is little information published on how effective TPE is on improving skin changes in general and no experience to date with the specific PPE protocol.
- All of the patients but one were currently using normal peripheral venous access to do TPE. TPE requires two IVs. The one used to withdraw blood is a larger needle that requires a good arm vein such as the antecubital. The return vein can be smaller. When normal peripheral venous access doesn’t work, ports are surgically implanted. This makes TPE very easy to do, but two commonly used ports (Vortex and PowerPort) cannot support blood flow at the rate normally achieved with arm veins so the entire procedure can take longer to do, often up to twice as long as a typical treatment which takes about 1 1/2 hours for a patient weighing about 150 pounds. There is actually one port that is FDA approved for doing TPE and that has the same or even higher flow rate than a normal arm vein, but it only has a single chamber so you still need a smaller arm vein for return when using this port. The patient with an implanted port is not using the FDA approved port so her TPE sessions are longer than they would be with arm veins.
- One interesting discussion was on how people managed to convince their doctor to try PPE. PPE has an excellent safety profile with only one known long-term side effect, mild iron deficiency anemia easily treated by over the counter iron supplements. It is much safer than any immunosuppressive medication commonly tried as a treatment for SSc, including methotrexate, Cellcept, Rituxan, so that helped a lot. And, at least in the US, most insurance companies now cover TPE as a treatment for SSc, including Medicare, so that was generally not a barrier. But, since many rheumatologists do not use TPE, they are often not knowledgeable about this and often have incorrect assumptions about safety and venous access. In several cases, patients backed their clinicians into a corner, asking them to give them a good reason for NOT trying PPE given that it is very safe and their insurance covered it. All of the patients were also very well educated about PPE so they could have informed discussions with their clinicians.
- As mentioned earlier, it is well documented in the research literature that patients undergoing TPE on a long-term basis will almost certainly develop mild iron deficiency anemia. It is mild enough so that over the counter iron supplements are able to deal with the mild anemia, but in patients who are unable to take oral iron supplements, iron infusions can easily be done in conjunction with TPE treatments as needed. One important fact that came out in the discussion is that a commonly used test to determine if someone has iron deficiency anemia – ferritin – is often normal in patients undergoing long-term TPE. However, another test – serum iron level – is usually low. In my own case as well as one of the newer patients, my clinician did a ferritin test that came back normal so I went off iron supplements. A few weeks later I became very fatigued and went back on iron, which eliminated the fatigue over a few weeks. The patient will be going back on iron supplements and hopefully this will help with some of her current fatigue symptoms.
- What happens if you stop PPE? In my own case, I went off my treatment plan for about six months back in 1997 to see what would happen. At that point, my GERD symptoms returned with a vengeance. It took almost a year after returning to the normal protocol to completely eliminate all of the GERD symptoms again (in contrast to about two years the first time with more severe GERD symptoms). Thanks to the current pandemic, we have more data on this key aspect of PPE. I stopped PPE for four months because of the pandemic and at that point my GERD was just starting to return but wasn’t bad yet. I immediately restarted the protocol and it took two complete four-treatment cycles over about 12 weeks to eliminate the mild GERD again. A second patient went off PPE for six months. In her case, her symptoms returned again at a significant level. She is back on the protocol but is now pregnant, which may or may not affect PPE treatment efficacy. We have zero data on this currently. My educated guess is that after she delivers, she will likely slowly return to full clinical remission over a nine month to one year time period, but that remains to be seen in practice. The best interpretation of what happens if you stop regular PPE treatments is that if you stop for around six months, you are basically starting over. Obviously, some symptoms that may have been slow to occur and slow to resolve, e.g., lung damage, won’t be back to pre-PPE levels over that short a time period, but it is clear that PPE needs to be continued in definitely to remain effective, assuming that it is effective at all. This is not at all surprising, of course, since SSc is a chronic disease that requires chronic treatment, whether with PPE or more typical treatments such as immunosuppressive meds.
I you have any questions, feel free to post them here and I will try to address them.
Ed Harris
Honorary Associate/Fellow (Rheumatology)
School of Medicine and Public Health
University of Wisconsin, Madison

The only published research looking at this was a case report. If you contact me directly at eharris@sclerodermainfo.org, I can send a copy to you. ESR is not a precise measure but it is well documented that it correlates highly with RBC aggregation levels.
Yes. I no longer actively monitor it but I frequently learn about patients now on pulsed plasma exchange around the world.
Are there more and more people trying PPE to date?
Ed,
I am really glad PPE worked for most people here, it would be interesting to know how ESR changes before vs after PPE.
Hi Ed,
As you know I have been diagnosed with systemic scleroderma in July after 7 months of trying to get a dx. I have lost 30 lbs and going downhill fast. I am suffering greatly with severe Gi discomfort including a choking feeling. In addition diffuse joint pain is occurring. I feel I can’t take another day of this. I am hoping to get PPE ASAP, before it’s too late. I wanted to know if you were able to connect with the doctors as discussed. I will await to hear from you. Thank you.
Newly diagnosed 19year old male high level athlete still waiting on first appointment with rheumatologist. Only obvious symptom currently is throat clearing. I am hoping to connect with you, Ed, prior to our appointment so that we can address the possibility of PPE with his rheumatologist.
Unfortunately there is not. But, if you have a doctor interesting in talking with another doctor who is doing this, I can directly connect them if they contact me.
hi ed,
is there an 9ngoing list of drs around the US who are using this protocol for treatment?
That is a really good question. I just sent an email to a few experts to find out. I will post what I learn about this.
My father is currently on dialysis as a result of SSc . Would this treatment be safe for him? I would like for him to try it. Please email me with the info.
I have responded to you directly by email.
I want to start treatment, I would like to have information about getting my physician on board with this treatment! Help!
Any treatment which can stop the disease process (potentially), for example autologous stem cell transplants, and possibly pulsed plasma exchange, allow the body to recover if the damage is reversible. It is likely that if skin changes are advanced or other organ damage is severe, it is very unlikely for these symptoms to improve. However, we know from observing what happens to the lungs when people stop smoking, for example, the body is gradually able to recover from a lot of damage over a long period of time. At this point, we don’t fully understand what will happen with treatments like these over time. We are seeing improvements in GI symptoms over time over a few patients, e.g., major recovery from GERD or improvements in GAVE (watermelon stomach) and also in one case improvements in lung functioning, but this was not a very advanced situation. Your point is a good one.
I can’t see how this program would help. The problem is skin type and tissue. The skin is not being replenished. Eventually it will harden. I soaked in oil, up to my neck, for 3 full days. It helped to make my stiff legs more pliable. It resulted in less skin pain and stiffness. When driving, my hands will seize up like a stroke but it is because my blood is not getting to the fingers when I keep them UP too long like more than 2 to 3 hours and I have problems walking after that time limit. The oil helped with mobility. Why is the skin not soaking in the oils that lubricate them? The other problem is that the blood is too acidic. The stomache seems to pour out extreme amounts of acids to cook down raw vegetables so I try to avoid them. Coke, coffee, tomatoes, wine….increases the pain within several hours which wakes me up. It seems to collect in my legs and arms. Lack of skin taking in oils and acid can not be changed by blood. Putting one drop or two, or less, in a ball of the male scrotum will possibly help with leukemia and it would have to be the same blood type but healthy. Will it help with a new type of skin? Too late for most of us. I was told I would be dead in 5 years after a cancer operation but I am still hanging in there. Keep safe during these times, iris