 I am currently (5/6/2022) at the American Society for Apheresis (ASFA) annual meeting in Philadelphia presenting a poster titled “The Effects of Pulsed Therapeutic Plasma Exchange on Gastrointestinal Symptoms in Limited Systemic Sclerosis: A Case Series” [1]. (The abstract of the poster is at the end of this post.) Six years ago, at this same conference, I presented an early version of the comprehensive review on the use of therapeutic plasma exchange as a treatment for systemic sclerosis that is now published in the “Journal of Scleroderma and Related Disorders” (http://dx.doi.org/10.1177/2397198318758606).
I am currently (5/6/2022) at the American Society for Apheresis (ASFA) annual meeting in Philadelphia presenting a poster titled “The Effects of Pulsed Therapeutic Plasma Exchange on Gastrointestinal Symptoms in Limited Systemic Sclerosis: A Case Series” [1]. (The abstract of the poster is at the end of this post.) Six years ago, at this same conference, I presented an early version of the comprehensive review on the use of therapeutic plasma exchange as a treatment for systemic sclerosis that is now published in the “Journal of Scleroderma and Related Disorders” (http://dx.doi.org/10.1177/2397198318758606).
While there are a number of patients now on the pulsed therapeutic plasma exchange (PPE) protocol described in this article (http://sclerodermainfo.org/pdf/TPE-Guidelines-US.pdf), the focus of this paper is on four patients who have the same antibody/diagnosis (centromere/limited systemic sclerosis), and who have been on this protocol for periods of time ranging from about 1 ½ years to more than 29 years.
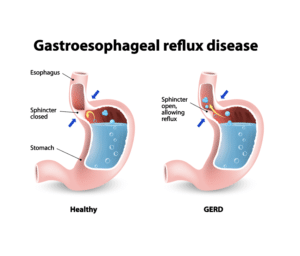
GI issues such as GERD are almost universal in patient with systemic scleroderma and many have additional GI symptoms including difficulty swallowing (dysphagia), delayed stomach emptying (gastroparesis), Gastric Antral Vascular Ectasia (GAVE) with blood loss, and Small Intestinal Bacterial Overgrowth (SIBO). Standard treatments such as immunosuppressive medications have no overall effect on GI symptoms, although many symptoms can be treated (with varying degrees of efficacy). While all four of these patients had significant improvements in other symptoms as well, what was striking was that all four also had significant improvements in GI symptoms including GERD and in one patient, GAVE.
Therapeutic plasma exchange (TPE) is commonly used to treat a number of disorders and has an excellent overall safety profile, with the only known long-term side effect being mild iron deficiency anemia (usually easily treated with over-the-counter iron supplements). The American Society for Apheresis classifies TPE to be a level III treatment when used as a treatment for systemic sclerosis, meaning that strong research such as recent clinical trials have not been done, but there is evidence suggesting that it may be effective and no contraindications. The best way to think about TPE as an experimental treatment for systemic scleroderma is as an “off label” label use of a standard treatment, as is also the case with medications such as methotrexate or mycophenolate mofetil (Cellcept), which are also not approved to treat systemic scleroderma.
Here are links to the poster itself and a much more detailed handout that will be used as the basis for an expanded version of this poster that will be submitted for publication in the near future:
Poster: ASFA-2022-Poster.pdf (sclerodermainfo.org)
Handout: ASFA-2022-Poster-Handout.pdf (sclerodermainfo.org)
Ed Harris
Founder/CEO
Scleroderma Education Project Ltd
===================================================================================
Abstract
Background: Up to 90% of patients with systemic sclerosis (SSc) experience significant gastrointestinal (GI) symptoms such as gastro esophageal reflux disease (GERD), small intestinal bacterial overgrowth (SIBO), and Gastric Antral Vascular Ectasia (GAVE) with associated anemia. Conventional systemic treatments including immunosuppression have no effect on the development of severe GI symptoms. Previous research on the use of therapeutic plasma exchange (TPE) as a treatment for SSc has typically described clear improvements in clinical symptoms and laboratory markers with very few adverse events. However, there has been almost no documentation on the effects of TPE on GI symptoms. This case series documents the effects on GI symptoms in four patients diagnosed with limited cutaneous systemic sclerosis (lcSSc) who have been on a specific pulsed plasma exchange (PPE) protocol for 15 months to 28 years.
Methods: All patients received a one blood volume TPE treatment per week for four weeks using albumin as the plasma replacement. This was followed by eight weeks with no treatment before the next cycle of four weekly treatments. Clinical assessment tools included the Scleroderma Health Assessment Questionnaire (SHAQ) and the UCLA Gastrointestinal Tract survey (GIT 2.0). No patients are on concurrent immunosuppressants.
Results: Data are summarized in Table 1. All four patients showed significant improvement in GI symptoms, including significant reduction or complete elimination of GERD in two patients, sustained normalization of hemoglobin in a patient with GAVE who had required six ablations and three iron infusions prior to starting PPE, and nearly complete elimination of severe esophageal spasms in a fourth patient. Two patients reported significant reduction of pain and fatigue, and in one patient, significant improvement was observed in diffusing capacity for carbon monoxide (DLCO). No significant adverse events related to TPE treatments were reported in any patient.
Conclusion: While all four of these patients exhibited significant improvements in a variety of clinical signs and symptoms, the observed improvements in a broad spectrum of GI symptoms is significant, since conventional treatments do not lead to GI symptom improvements. Given that GI involvement can severely affect quality of life in patients with SSc and that previous research has demonstrated that TPE has an excellent safety profile, these preliminary results suggest that pulsed plasma exchange should be considered in lcSSc patients with significant GI symptoms. Additional research is needed to better understand the mechanisms of action for this treatment modality and whether they will replicate in SSc patients with different antibodies and clinical profiles.
(Note: Table 1 is included in the detailed Handout.)
——————————————————————-
1. Harris, E., Weiss J., Lacson S. (2022). The Effects of Pulsed Therapeutic Plasma Exchange on Gastrointestinal Symptoms in Limited Systemic Sclerosis: A Case Series. J Clin Apher, 37(2), 168.

This is a great and well thought out question. ESR is a complex measure. On average it is elevated in patients with Scl-y0 antibodies and slightly elevated in patients with centromere antibodies, both most patients have ESR results within the normal range. The few studies that have directly examined the blood of SSc patients has documented that RBCs are clumped together to some extent. The cause of the clumping is not currently understood and most of the clumps are small, but they are tightly bound, shear-resistant clumps, not like Rouleaux formations, which break apart and reform quickly. But here is the complication. If you have clumped RBCs in normal plasma, you will have a higher sed rate than if your RBCs were not clumped. But, if the plasma concentration is elevated, that can override the clumped RBCs and that can lead to very low ESR values. This has been studied in rheumatoid arthritis patients, but not SSc patients. The most likely explanation is that you have plasma that has elevated viscosity. I do not know what causes this or the clinical significance, but that is the mostly likely explanation.
Ed,
fascinating work.
if I understand the hypothesis correctly , the rbc clumping is the root cause of many symptoms in SSc, and PPE will break this clumping, hence resolve these symptoms.
as a proxy test of rbc clumping, my ESR-Wes is 2 mm/hr, the reference range is 0-30 (labCorp), so it is normal. CRP is also completely normal, does this mean I don’t have abnormal rbc clumping, and PPE will not help me ?
My scl-70 is 7.1, normal range is below 0.9. But I don’t have Renault yet.
There are some patients with diffuse using the protocol, but we have less data on most of these patients than we do for patients with centromere antibodies. One patient with very early diffuse with a rare antibody (U3-RNP) went on the protocol when her major symptoms were severe pain and fatigue, common in early diffuse. She went into full remission after about nine months and except for when she stopped the protocol (during COVID) and regressed partially, she has been in full remission for several years now. But, she started quite early without major damage to organs that would be hard or impossible to reverse. I am now suggesting that diffuse patients use a slightly more aggressive protocol for at least the first year as the disease progresses more rapidly than in patients with limited or overlap variants. Instead of the normal eight week gaps between treatment cycles, I am suggesting a six week gap for the first year. If the treatment appears to be working well, it is worth slowing trying to taper down to the typical eight week gap over the next year to see if that will maintain benefit.
Ed,
Are there any studies of TPE with people who have diffuse systemic scleroderma?