Background
Over the past 42 years, many published papers have documented that blood rheology is abnormal in patients with systemic sclerosis (SSc). Individual papers have commented on or measured differing aspects of this abnormal rheology, including elevated whole blood viscosity (WBV), increased plasma viscosity (PV), decreased red blood cell (RBC) deformability, and abnormal RBC aggregation* [1-17].
Beyond the research showing elevated whole blood viscosity, many SSc patients that were blood donors note anecdotally that at the point that they started to develop SSc-related symptoms, the lab techs drawing blood commented that their blood was noticeably thicker than normal and that it took much longer than expected to do the blood draw. (It is worth noting that diagnosed SSc patients cannot be blood donors. However, as it can sometimes take years for patients to finally receive a correct diagnosis, patients will often continue to be blood donors during that pre-diagnostic period.) In addition, some patients were told that their blood was clogging the filters in the equipment used to extract blood products from the donated blood. According to a well-known SSc researcher and clinician, the hyperviscosity “actually can be visualized as sluggish flow through a nailfold video capillaroscopy”.
Why is this significant? In 1979, Kahaleh et al. [18] noted that,
“Many, if not all, of the manifestations of scleroderma can be explained on the basis of functional and structural vascular compromise after repeated vascular insults, subsequent healing of vascular walls with proliferative vascular response, and luminal narrowing.”
This is still a commonly accepted viewpoint [19]. Several different potential mechanisms for this initial endothelial damage have been proposed, including viral triggers, cytotoxic T-cell involvement, and anti-endothelial antibodies [20]. However, none of these proposed endothelial damage mechanisms have been consistently demonstrated to be universal in SSc. For example, anti-endothelial antibodies are not universally found in patients with SSc and are also found in other autoimmune diseases, including SLE, RA, and Sjögren’s syndrome [21].
* See discussion below on what is meant by “RBC Aggregation”
Hypothesis
Abnormally clumped red blood cells may be a significant component of the etiopathogenic processes in SSc, potentially contributing to the vascular damage cited above.
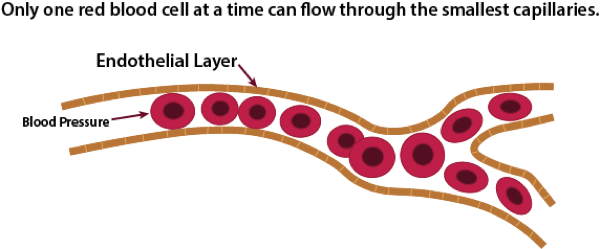
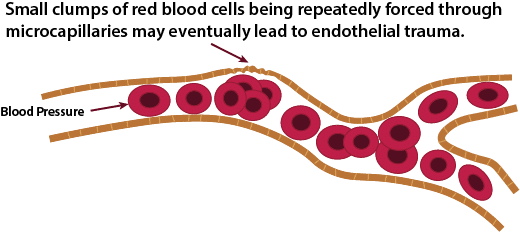
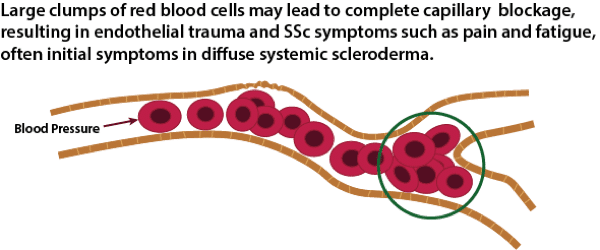
The average size of a micro-capillary is about 8 microns in diameter, with a normal range of 5 to 10 microns. A normal red blood cell is 6 to 8 microns in diameter. This means that some RBCs have to fold in order to fit through the smallest capillaries. As RBCs start to clump together, it becomes increasingly difficult for these clumps to make it through the smallest micro-capillaries. Normal blood pressure is very strong, and at least for a while, the pressure will be strong enough to force the clump through the micro-capillaries. However, at some point, this may potentially start to cause damage to the single layer of endothelial cells that line the micro-capillaries. The research literature on the effects of clumped RBCss on micro-capillaries documents most of the early symptoms seen in SSc, including tortuous capillaries that are seen in nail beds and glomerular damage (kidneys) caused by hemodynamic mechanisms [22,23,24]. The exact mechanisms of endothelial damage from clumped RBCs are currently unknown, but likely include direct mechanical effects tending to remodel vessel walls and changes due to local ischemia caused by abnormal distribution of red cells in the microcirculation [25].
Normal Blood Flow
Endothelial Damage
Microcapillary Blockage
Although entirely speculative, a reasonable and testable hypothesis would be that with limited cutaneous systemic sclerosis (lcSSc), either the degree of RBC clumping or the “stickiness” of the RBC clumps may be lower than in more rapidly progressing diffuse cutaneous systemic sclerosis (dcSSc). There is some support for this hypothesis in the research literature (see discussion below in the section below: Hyperviscosity Characteristics for Different Antibody Types). Even if the degree of clumping is low but constant, and the overall hyperviscosity of the blood is only slightly elevated, over a long period of time damage to the endothelial lining could still occur resulting in the development and progression of SSc symptoms.
RBC clumping can also potentially explain one of the common very early symptoms experienced by many SSc patients – severe fatigue. This is more commonly seen as an initial symptom in patients with dcSSc than it is in patients with lcSSc. The reasoning behind this speculation is straight forward: tightly clumped RBCs could cause functional anemia, even though this would not be obvious with normal blood tests such as hematocrit and hemoglobin. The clumped RBCs would be functionally similar to sickle cells seen in patients with sickle cell anemia – the clumping of sickle cells results in greatly impaired blood flow. Notably, some of the common symptoms of sickle cell anemia include fatigue, shortness of breath, and cold hands and feet. These are common early SSc symptoms, especially in patients with diffuse SSc.
What is “RBC Aggregation”?
Before looking at the data that in support of this disease pathogenesis hypothesis, it is important to understand exactly what is meant by the term “RBC aggregation”, as used in the various papers describing this phenomenon in patients with SSc. Unfortunately, the term “RBC aggregation” is often used in the research literature as a generic term for RBCs that are clumped together regardless of the nature of and cause of the clumping.
There are two known ways that RBCs clump together (it is possible that there are others). The first is the reversible clumping of RBCs into linear stacks call rouleaux, which form because of the discoid shape of vertebrate RBCs. The discoid shape gives RBCs a large surface area for making contact and sticking to each other. Fibrinogen and other acute-phase proteins interact with sialic acid on the RBC surface, which facilitates rouleaux formations. Consequently, various infections, neoplasms, and inflammatory and connective tissue disorders are accompanied by enhanced rouleaux formations. Also, rouleaux are one of the causative factors of diabetic retinopathy [26] RBCs aggregation into rouleaux is a relatively fast process, usually occurring within seconds. High shear forces easily break apart this type of aggregation, only to reform again quickly at stasis or in low shear conditions [27].
The second known way that RBCs clump together is through a process called agglutination. This is typically caused by antibodies (immunoglobulins), which cross-link and aggregate RBCs by binding to corresponding surface epitopes on adjacent RBCs. One common example of this occurs in cold agglutinin disease (CAD), which is a rare form of hemolytic anemia caused by antibodies, which bind to RBCs only at lower-than-normal body temperatures, typically below 32 degrees of Celsius. Most forms of CAG are the result of high circulating concentrations of IgM antibodies binding primarily to I/i antigens on the RBC surface. However, one variant of CAD – Donath-Landsteiner Hemolytic Anemia (DLHA) – is caused by a smaller IgG antibody binding to the I or P antigen. The binding of IgM or IgG antibodies to RBCs can activate the classical pathway of complement, resulting in the formation of the membrane attack complex, which creates pores in the membrane of RBCs causing their lysis. Also, opsonizing complement molecules induced by the activation of complement greatly facilitate phagocytosis of opsonized RBCs by macrophages. Thus, these two processes of direct lysis and phagocytosis of RBCs are the causative mechanism of CAD [28]. Notably, IgG antibodies bind more easily to older RBCs than younger ones [29].
Unfortunately, none of the published papers that mention RBC aggregation in SSc directly address the issue of what type of RBC aggregation is being observed/measured. However, it is unlikely that the sole mechanism of RBC aggregation (using “aggregation” in the generic sense) in SSc is rouleaux formation for two reasons:
- Rouleaux stacks form very rapidly in low shear and stasis situations but break apart easily in high-shear environments. A 1991 paper measured RBC aggregation levels before and after a single round of four weekly TPE treatments and noted that the normalized blood rheology, including elimination of RBC aggregation, lasted for about six months before returning to baseline levels [1]. This would not be expected if rouleaux formation was the sole method of RBC aggregation in SSc.
- One early paper [30] focused on changes in hemorheological changes in SSc following TPE notes the “abnormally high ability to withstand the flow forces in vitro” of the observed RBC aggregates. This would not be consistent with simple rouleaux formation.
Implications for Treatment
If the hyperviscosity damage model for microcapillary endothelial damage is correct, then this presents an opportunity for a stage 3 intervention (see Disease Staging model discussion: SSc Disease Staging). In theory, any treatment that can reduce or eliminate RBC clumping should reduce or prevent the development of SSc-related symptoms.
Therapeutic Plasma Exchange
Therapeutic Plasma Exchange (TPE; also called plasmapheresis) is considered the “gold standard” in treating blood hyperviscosity disorders [31]. Basically, TPE is a procedure that mechanically replaces most of the plasma while preserving blood cells. Specifically, the procedure involves removing blood from one arm, running it through a machine that filters out and keeps the red and white blood cells and most of the platelets, discards the plasma (the liquid part of the blood), and replaces it with either new plasma or, more commonly, sterilized albumin. The combined albumin and the original red and white cells plus platelets are remixed and returned to the other arm. Typically, this takes about 90 minutes and is done in an outpatient hospital environment. The effect of a single one blood volume plasmapheresis treatment is to remove about 65% of blood components except for almost all of the red and white blood cells and about 70% to 75% of the platelets. This includes beneficial things like clotting factors but also potentially harmful things such as autoantibodies.
There is a full discussion of the research literature on the use of TPE as a treatment for SSc in the Therapeutic Plasma Exchange section). It is notable, however, that even though both rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) show abnormal blood rheology including increased RBC aggregation [32,33], TPE is not effective in either of these diseases [34,35].
TPE and SSc Blood Rheology
In the mid 1980s through the early 1990s a series of investigational research studies [1,30,36,37] were done in the Netherlands that examined red blood cell aggregation in patients with primary Raynaud’s (not related to an underlying autoimmune condition) and patients with secondary Raynaud’s associated with systemic sclerosis. They developed a way of measuring blood viscosity in vivo (live circulation) rather than in vitro (in a test tube). In vivo testing is considered a more reliable way of measuring viscosity.
The initial studies looked first at the difference in blood viscosity between these two groups of Raynaud’s patients. The researchers then tried using therapeutic plasma exchange on both the primary and secondary Raynaud’s patients. The treatment protocol in these early studies mostly involved doing four TPE treatments – one per week for four weeks – and then studying the results of this intervention.
Here is a summary of some of the key findings from this series of research studies:
- While overall blood viscosity was slightly elevated in patients with primary Raynaud’s (versus normal controls) and even more elevated in secondary Raynaud’s patients, when they looked specifically at RBC aggregation, they found that RBC aggregation was the same for the control group and primary Raynaud’s patients, but highly elevated for the secondary Raynaud’s patient group. This finding suggests that different mechanisms are likely to be involved in the two different forms of Raynaud’s.
- The four-treatment intervention protocol (one treatment per week for four weeks) essentially eliminated the abnormal RBC aggregation found in the secondary Raynaud’s patient group. What they also found was that this treatment protocol had almost no effect on Raynaud’s symptoms in patients with primary Raynaud’s. In contrast, this treatment regimen typically eliminated all of the Raynaud’s symptoms in the SSc patients with secondary Raynaud’s for six months or longer.
- The studies also reported significant improvement in other SSc-related symptoms, including healing of digital ulcers. Patients were monitored for up to three years following this single series of treatments. After a varying number of months, RBC aggregation returned to pre-treatment levels and Raynaud’s symptoms redeveloped, but none of the patients developed skin ulcers during the three-year follow up period in one study [1].
Alternative Treatment Approaches
While therapeutic plasma exchange is the only specific SSc treatment intervention that has been documented to improve abnormal SSc blood rheology in a research setting, there are several alternative treatment approaches to reducing red blood cell clumping that should be studied. It is important to note that these alternative approaches to reducing red blood cell clumping have not been tested with SSc patients, so even though there is some limited research indicating that these treatments appear to reduce RBC aggregation, these findings may not generalize to SSc-specific RBC clumping.
Pharmaceutical Approaches to Treating SSc Hyperviscosity
Most pharmaceutical interventions for hyperviscosity syndromes target forms of hyperviscosity that results from excess plasma proteins, white blood cells, or platelets instead of the elevated whole blood viscosity and RBC aggregation that research indicates is the case with SSc patients. Because of this, initial drug trials should focus on drugs that specifically have been shown to reduce RBC aggregation:
- Nattokinase is a fibrinolytic enzyme (compound that dissolves blood clots) that is extracted from natto – a vegetable cheese-like food made from fermented soybeans that has been popular in Japan for more than 1000 years. Nattokinase was isolated from Natto in 1980 by Dr. Hiroyuki Sum at the University of Chicago Medical School. A number of studies, both in vivo and in vitro, have consistently shown that nattokinase has strong pro-fibrinolytic beneficial effects, including delaying clotting, platelet aggregation and the development of blood clots. In addition, a recent study [38] looked at the effects of nattokinase on RBC aggregation. This study showed dose dependent decrease in RBC aggregation as well as overall plasma viscosity. Nattokinase is sold as an over-the-counter supplement in the US but is considered a drug and less available in some other countries. While nattokinase is generally considered safe, it can interact with other medications, for example, anticoagulant drugs such as warfarin and heparin, and should only be used under medical supervision.Since nattokinase is widely available in the US as an over-the-counter supplement at a very low price, it would be relatively easy and inexpensive to conduct an initial research study to look at its safety and efficacy in a small group of SSc patients. An open label pilot study could look at the effects of daily administration of two different dosage levels over a limited time period (3 to 6 months). The primary dependent measure would be changes (if any) in total blood viscosity and RBC aggregation. If no reduction of RBC aggregation is evident after an initial 3-month trial period, then nattokinase is unlikely to be effective in treating SSc.
- Vepoloxamer™ (LifeRaft Biosciences) is an investigational drug with potential utility in a wide range of diseases that are characterized by impaired blood flow. The active ingredient in Vepoloxamer is a purified form of poloxamer 188. Research has shown that poloxamer 188 lowers blood viscosity, decreases RBC aggregation, and decreases friction between RBC and vessel walls to increase microvascular blood flow and decrease cell injury [39]. Poloxamer 188 is considered safe and is widely used in many over the counter products such as toothpaste, laxatives, and mouthwash. The FDA approved it more than 50 years ago as a therapeutic reagent to reduce viscosity of blood before it is used in transfusions. While it has mostly been studied as a potential treatment for sickle cell disease, it may also have potential to treat SSc by reducing RBC aggregation, inflammation, and restoring cell membrane integrity.
Non-pharmaceutical Approaches to Treating SSc Hyperviscosity
Laser Blood Irradiation Therapy – There is some research that suggests that Intravenous Laser Blood Irradiation Therapy can temporarily reduce RBC aggregation and improve RBC deformability in vitro [40]. Since this effect only lasts for a few hours, it would presumably require frequent treatments in order for this approach to potentially be effective in treating SSc patients. While this is clearly not practical with an intravenous approach, a Canadian company (Vielight, Inc.) has developed an intranasal device that they claim can achieve the same results as intravenous irradiation using a device which irradiates circulating blood through the inside of the nasal cavity.
Because of the uncertainty of whether the short duration of decreased RBC aggregation is likely to be effective in SSc patients, an initial pilot study should be limited to a small group of patients in order to determine if this approach is safe and effective.
Hyperviscosity: Directions for Future Research
Endothelial Damage – Biomechanical or Biochemical
One of the major unanswered questions in SSc research is whether the antibodies associated with SSc actually cause the development of SSc related symptoms (pathogenic theory) or instead are merely a marker of the underlying disease (epiphenomena theory). (Note: see the Antibody section of the Scleroderma FAQ for a complete list of currently identified SSc-specific antibodies: Systemic Sclerosis Antibodies.) The above-mentioned research on hyperviscosity, endothelial damage, and TPE is consistent with the pathogenic theory but is not definitive. A single TPE treatment of 1 to 1.5 blood volumes removes approximately 65% of any potential circulating pathogenic factors [41]. This includes SSc-specific antibodies (as well as beneficial antibodies) but also might include co-factors also circulating in the blood that are actually the direct cause of the red blood cell hyperaggregation.
The hypothesis advanced in this paper is that abnormally clumped RBCs may be a significant component of the etiopathogenic processes in SSc, potentially causing or contributing to the vascular damage to the endothelial layers of the microcapillaries that appear to trigger the downstream fibrotic processes and systemic fibrosis that are the hallmark of SSc. The body of research that shows that a short series of TPE treatments eliminates RBC aggregation for a significant period of time is consistent with this hypothesis but does not directly answer the question as to whether or not the hypothesis is correct. It can reasonably be argued that the fact that TPE also temporarily eliminates a significant percentage of circulating (potentially pathogenic) autoantibodies or some other cofactor is also consistent with an alternative hypothesis that it is simply the reduction in the number of circulating antibodies or other potential pathogenic molecules that is somehow leading to the symptom improvement.
Support for RBC Aggregation Endothelial Damage Hypothesis
Therapeutic Plasma Exchange
While certainly far from definitive, some of the results from the series of early TPE treatment studies do appear to support the RBC hyperaggregation pathogenesis hypothesis over the alternative temporary reduction of circulating pathogenic factors hypothesis. One of the more striking findings in many of the published studies on the use of TPE to treat patients with SSc is that a series of four weekly TPE treatments quickly eliminated Raynaud’s symptoms from almost all of the test subjects, including patients with long standing Raynaud’s secondary to SSc. With alternative treatments that also reduce the number of circulating antibodies, e.g., immunosuppressant therapies, this rapid elimination of Raynaud’s symptoms does not occur.
Raynaud’s symptoms occur from the blood vessels spasming and dramatically restricting blood flow for a period of time. The early research on TPE and Raynaud’s demonstrated that there is almost no RBC aggregation in patients with primary Raynaud’s (in contrast to those with Raynaud’s secondary to SSc). It also showed that TPE had little effect on primary Raynaud’s symptoms but was very effective in reducing secondary Raynaud’s symptoms.
This suggests very different mechanisms of action in the two different forms of Raynaud’s. It is entirely reasonable that if the endothelial damage is caused by clumped RBCs, this could easily lead to hyper-sensitive blood vessels that spasm and restrict blood flow, resulting in classic Raynaud’s symptoms. However, if RBC clumping is eliminated, you would expect that healing would occur fairly rapidly since there would be no more trauma to the endothelium and this would be likely to reduce or eliminate the Raynaud’s symptoms. In contrast, any treatment that gradually reduces the number of circulating autoantibodies might result in a corresponding gradual improvement in Raynaud’s symptoms, but would not occur as rapidly.
One of the earliest studies of using plasma exchange for treating patients with Raynaud’s syndrome [42] could not explain why short-term reduction in presumed circulating pathogenic factors from one series of four weekly TPE treatments could lead to long-term symptomatic and quantitative improvements. Since later research has shown that a single four-week TPE treatment series results in elimination of RBC aggregation for a typical six-month or longer time period [1], this lends support to the hypothesis that direct mechanical elimination of RBC clumping through centrifugal separation accounts for long-term systemic improvements rather than the more indirect effective of temporarily reducing potential circulating pathogenic factors. However, this interpretation of the longevity affect requires an RBC clumping model that is based on some type of binding beyond simple, temporary Rouleaux formation, for reasons stated earlier.
Endothelial Damage from Clumped or Enlarged RBC
While the precise etiology of SSc has not been fully established, research indicates that both genetic susceptibility and some type of (usually) environmental trigger are required for the development of the disease [43]. However, our disease model predicts that any disease which has high-shear resistant RBC clumping should lead to endothelial damage.
Sickle cell disease (SCD) is characterized by misshapen RBCs leading to abnormal RBC aggregation. Increased shear forces are needed to disperse RBC aggregates, potentially leading to disturbed blood flow at the microcirculatory level since aggregated RBC are unable to pass through the smallest microcapillaries that normally allow only single RBC to pass through [44]. Vasoocclusion in SCD causes tissue ischemia and endothelial injury [45]. While SCD is clearly different from SSc in many ways, there are some symptom parallels, including early onset pain and fatigue in SSc that is similar to what is seen in sickle cell anemia crisis.
Testing the RBC Aggregation Endothelial Damage Hypothesis
Autologous TPE
There is, in fact, a fairly easy way to do the research needed to determine if the improvement in SSc-related symptoms seen with TPE is a function of the mechanical separation of clumped RBCs or more a result of temporarily reduced antibody (or other potential circulating pathogenic molecule) levels. Basically, any alternative treatment approach that: 1) reduces or eliminates RBC aggregation for a period of time, and 2) leads to an objective reductive in SSc-related symptoms without reducing overall circulating levels of antibodies or other pathogenic factors, then this would lend strong support to the hypothesis that the endothelial cell damage is, in fact, a direct result of damage triggered by clumped RBCs rather than the reduction of circulating pathogenic substances.
Normal TPE treatments work as follows:
- Blood is continuously extracted and run through a centrifuge that separates out red blood cells, white blood cells, and platelets from the plasma. This results in separation of any clumped RBCs. This disaggregation effect may be a direct result of the mechanical process of separating out the red cells from the plasma, or this could alternatively be the result of the removal of a plasma component that is leading to the clumping, thus allowing the RBC to separate.
- The plasma (containing SSc-related and other antibodies) is discarded.
- The extracted red cells, white blood cells, and platelets are combined with either sterilized albumin or donated plasma and re-circulated back to the patient.
Instead of discarding the patient’s plasma and replacing it with new autoantibody-free donated plasma or sterilized albumin, it is actually very easy to modify the plumbing on the equipment used to perform TPE to instead recombine the patient’s original plasma with the cells that have been separated out by the centrifugal separation process. These red cells would no longer be clumped, but since the original plasma is maintained, the level of circulating autoantibodies and other potential pathogenic factors would not be reduced, as is the case with normal TPE treatments. This procedure is known as autologous TPE. If Raynaud’s symptoms are eliminated or significantly improved using this altered TPE intervention, then this would strongly support the hypothesis that the improvement was a result of the elimination of RBC clumping rather than a reduction in circulating pathogenic molecules.
It turns out that a very early pilot study that used TPE to treat a small group of patients with SSc and Raynaud’s used autologous plasma exchange as a control group with the expectation that it would act as a placebo control treatment [46]. However, the researchers were surprised that improvements in pulse volume, digital blood pressure, and skin temperature occurred in some of the patients with both the standard plasma exchange procedure and also with “placebo” plasma exchange. Since both regular and autologous TPE mechanically disaggregate RBCs, this result is not surprising if RBC disaggregation is the primary mechanism of action from TPE treatments rather than reduction in circulating antibody levels.
Extracorporeal Photopheresis
One other area of research on SSc treatments may lend additional indirect support to the disaggregation hypothesis. A number of [47,48,49,50,51] have looked at using extracorporeal photopheresis (ECP) as a way to treat SSc patients. Extracorporeal photopheresis is used primarily to treat T-cell lymphomas or diseases such as graft-versus-host disease that can occur with organ transplants. The process is similar to TPE in that the patient’s blood is withdrawn intravenously, cells are separated out by mechanical centrifuging, and the extracted white blood cells are treated with a drug called psoralen which makes T-lymphocytes more sensitive to ultraviolet light. The treated cells are then treated with UV light, causing death of diseased cells, and then returned to the patient’s body. In almost all studies of ECP for treating patients with SSc, photopheresis has been found to be beneficial, although many of the studies were pilot studies done with patients having different specific SSc diagnoses or stages of the disease process. In addition, the outcome measures varied widely among the various studies.
What is particular noteworthy is that in all of these studies, you will see a statement such as “the mechanism by which photopheresis is of therapeutic benefit in SSc is unclear at present” [51]. While a few of these studies offer (highly creative) hypotheses that might account for some of the beneficial effects, it is very clear from reviewing these studies that the researchers really have no idea why ECP led to symptom improvements. However, if the beneficial effects of ECP are viewed from the perspective of disaggregating clumped RBCs, the observed therapeutic benefits make complete and logical sense since photopheresis, like TPE, uses a centrifugal blood separation process that should lead to the same type of mechanical disaggregation of RBCs that has been documented with TPE. More important, photopheresis does not reduce circulating antibody levels, in essence making ECP somewhat analogous to the proposed autologous TPE approach outlined above and adding support to the hypothesis that most of the beneficial effects of TPE are from RBC disaggregation instead of temporary reduction of circulating pathogenic factors.
Potential Additional Benefit from Reduction of Circulating Pathogenic Factors?
While a major hypothesis advanced in this paper is that the primary benefit seen from treating SSc patients with TPE may be from RBC disaggregation rather than from temporary reduction of potential circulating pathogenic factors such as autoantibodies, it is important to note that if the circulating molecules are in fact pathogenic, it would be expected that temporarily reducing the levels of potential circulating pathogenic molecules using standard TPE might result in a longer-lasting beneficial therapeutic effect then would occur using autologous TPE or similar procedures that simply disaggregate RBC. Early TPE studies that studied the impact of TPE treatments on Raynaud’s symptoms indicated that the Raynaud’s symptoms generally did not reappear for at least several months following a series of four weekly standard TPE treatments. It is certainly possible that any RBC clumping would reoccur more rapidly with autologous TPE treatments, resulting in a return of Raynaud’s symptoms in a shorter period of time. However, since the primary goal of a controlled study comparing the effects of standard versus autologous TPE treatments on RBC aggregation would be to determine whether or not the elimination of Raynaud’s symptoms is mostly a function of RBC disaggregation or instead from reducing circulating potential pathogenic factor levels, the duration of the response is not of paramount importance in determining the exact mechanisms of action of TPE. However, if it can be shown that there is a clear benefit to using a procedure that temporarily reduces levels of potential pathogenic factors in addition to disaggregating clumped RBCs, then the higher cost of standard TPE as compared to autologous TPE may be justified in some cases.
Hyperviscosity Characteristics for Different Antibody Types
Basic initial research needs to be done to look at the blood rheology characteristics of SSc patients with different antibody variants, including the degree of RBC clumping (if abnormal), RBC deformability, and the “stickiness” of the aggregated cells. It is possible that different antibody subtypes might have different aggregation properties. For example, even if two different subtypes show a similar degree of clumping when the heart is resting (diastolic blood pressure), if one subtype is more “sticky” than the other subtype, then when the heart is pumping (systolic blood pressure), those clumped RBCs would be less likely to break apart – potentially causing more damage to the endothelial cells.
A recent paper [52] lends indirect support to the hypothesis that different variants of SSc may have different degrees of RBC aggregation. While the main focus of this article was on the association between C-Reactive Protein (CRP) and disease activity in SSc, it included data showing that patients with diffuse cutaneous systemic sclerosis (dcSSc) had much higher erythrocyte sedimentation rates (ESR) than patients with limited cutaneous systemic sclerosis (lcSSc). ESR is highly correlated with RBC aggregation and can thus be considered to be strong proxy measure of RBC aggregation [53]. While speculative at this point, this could potentially explain some of the differences in symptom development between lcSSc and dcSSc patients. If RBC aggregation consists of smaller and fewer clumps in patients with in lcSSc variants, then this could lead to slower rates of endothelial damage and subsequent slower development of Raynaud’s and other symptoms. In contrast, with dcSSc variants, early symptoms often include severe fatigue and pain. If the RBC aggregation in dcSSc is characterized by larger clumps of RBC, this could directly lead to capillary blockage similar to what is seen in sickle cell anemia crisis, which shares similar symptoms of anemia-based fatigue and pain from the directly impaired circulation.
The Muangchan paper [52] also indicates that ESR (RBC aggregation by proxy) is higher in early stages of dcSSc (< 3 years) than it is in later stages (> 3 years). Often, but not universally, dcSSc patients show spontaneous reduction in modified Rodnan skin thickness scores (MRSS) after an initial period one top two years of rapid skin changes [54]. If the overall model advanced in this paper is correct – that RBC aggregation is a major component of the vascular damage seen in SSc – then this later reduction in ESR/RBC aggregation could provide one possible explanation for this phenomenon.
Any studies of hyperviscosity characteristics should be separated into antibody specific patient subsets. Initially, anti-centromere (ACA) positive and anti-Scl70 patient groups are the most easily identified for research purposes. Ideally, patients with anti-RNA Polymerase III antibodies should also be studied as an additional subtype since research suggest that this antibody profile occurs with a similar frequency to ACA and anti-Scl70 antibodies.
Note: there are known false positive issues with Scl-70 antibody testing using ELISA and Multiplex testing [55]. If the patient is diagnosed with dcSSc with positive Scl-70 antibodies based on ELISA or Multiplex testing, we suggest that more reliable testing methods such as immunodiffusion be used to confirm this antibody result.
Issues with Blood Rheology Testing
Any studies looking at blood rheology in SSc should ideally examine a number of different factors, including:
- Overall blood viscosity
- Plasma viscosity
- Red blood cell clumping/aggregation
- Red blood cell deformability
A number of companies make equipment for measuring whole blood and plasma viscosity. This includes Health Onvector (SCV-300™) and RheoSense (m-VROC™). RBC aggregation and RBC deformability can be measured by systems such as the LORCA™ (Laser-assisted Optical Rotational Cell Analyzer) from RR Mechatronics.
One major issue that needs to be considered in any studies looking at blood rheology is sample longevity. While it is possible to ship blood samples overnight to a central lab for rheology testing, in practice this is very expensive and also requires careful timing and coordination between the facility doing the blood draw and the receiving lab that has the equipment for blood rheology testing. An alternative possibility that should be considered in some studies focused on examining blood rheology in a large group of SSc patients would be to ship the testing equipment to a suitable location near a large, potential patient/subject pool, conduct all testing over a two or three-day period, and then return the equipment to the central lab or to an additional location if needed. While equipment shipping, setup cost, and staff travel expenses is significant, it is likely to be less expensive than overnight shipping of a large group of samples and will also be much more likely to minimize any issues with blood sample viability from handling and shipping problems.
References
- Jacobs MJ, Jörning PJ, Van Rhede van der Kloot EJ, et al. Plasmapheresis in Raynaud’s phenomenon in systemic sclerosis: a microcirculatory study. Int J Microcirc Clin Exp. 1991;10(1):1-11.
- Dodds AJ, O’Reilly MJ, Yates CJ, Cotton LT, Flute PT, Dormandy JA. Haemorrheological response to plasma exchange in Raynaud’s syndrome. Br Med J. 1979;2(6199):1186-1187.
- Hamilton W, White J, Cotton L. Circulatory improvement in Raynaud’s phenomenon following plasma exchange. In: Sieberth HG (Ed) Plasma Exchange. Stuttgart New York: Schattauer; 1980:301-307.
- Talpos G, Horrocks M, White JM, Cotton LT. Plasmapheresis in Raynaud’s disease. Lancet (London, England). 1978;1(8061):416-417.
- Weber H, H S-S, J LHA. Plasmapheresis as a Treatment of Raynaud’s Attacks: Microrheological Differential Diagnosis and Evaluation of Efficacy. Clin Hemorheol Microcirc. 1985;5:85-97.
- Blunt RJ, George AJ, Hurlow RA, Strachan CJL, Stuart J. Hyperviscosity and Thrombotic Changes in Idiopathic and Secondary Raynaud’s Syndrome. Br J Haematol. 1980;45(4):651-658. doi:10.1111/j.1365-2141.1980.tb07188.x.
- Dintenfass L. Hemorheological factors in Raynaud’s phenomenon. Angiology. 1977;28(7):472-481.
- Ernst E, Lohmaier EF, Meurer M, Gerstmeier J. [Decreased blood fluidity in progressive systemic scleroderma]. Z Rheumatol. 1990;49(3):155-159.
- Jacobs MJ, Breslau PJ, Slaaf DW, Reneman RS, Lemmens JA. Nomenclature of Raynaud’s phenomenon: a capillary microscopic and hemorheologic study. Surgery. 1987;101(2):136-145.
- Lacombe C, Mouthon JM, Bucherer C, Lelievre JC, Bletry O, Godeau P. [Raynaud’s phenomenon and blood viscosity]. J Mal Vasc. 1992;17 Suppl B:132-135.
- Larcan A, Schmidt C, Stoltz JF, Voisin P. [Blood rheology in Raynaud’s disease]. J Mal Vasc. 1984;9(1):1-6.
- McGrath MA, Peek R, Penny R. Blood hyperviscosity with reduced skin blood flow in scleroderma. Ann Rheum Dis. 1977;36(6):569-574.
- Picart C, Carpentier PH, Brasseur S, Galliard H, Piau JM. Systemic sclerosis: blood rheometry and laser Doppler imaging of digital cutaneous microcirculation during local cold exposure. Clin Hemorheol Microcirc. 1998;18(1):47-58.
- Rustin MH, Kovacs IB, Sowemimo-Coker SO, Maddison PJ, Kirby JD. Differences in red cell behaviour between patients with Raynaud’s phenomenon and systemic sclerosis and patients with Raynaud’s disease. Br J Dermatol. 1985;113(3):265-272.
- Tietjen GW, Chien S, Leroy EC, Gavras I, Gavras H, Gump FE. Blood viscosity, plasma proteins, and Raynaud syndrome. Arch Surg. 1975;110(11):1343-1346.
- Vayá A, Todolí J, Calvo J, Romagnoli M, Ricart JM. Haemorheological profile in patients with systemic sclerosis. Clin Hemorheol Microcirc. 2008;40(3):243-248.
- Korsten P, Niewold TB, Zeisberg M, et al. Increased Whole Blood Viscosity Is Associated with the Presence of Digital Ulcers in Systemic Sclerosis: Results from a Cross-Sectional Pilot Study. Autoimmune Dis. 2017;2017:1-5. doi:10.1155/2017/3529214.
- Kahaleh MB, Sherer GK, LeRoy EC. Endothelial injury in scleroderma. Journal of Experimental Medicine. 1979; 149(6); 1326-1335.
- Matucci-Cerinic M, Kahaleh B, Wigley FM. Review: Evidence That Systemic Sclerosis Is a Vascular Disease. Arthritis Rheum. 2013;65(8):1953-1962. doi:10.1002/art.37988.
- Kahaleh B. Vascular Disease in Scleroderma: Mechanisms of Vascular Injury. Rheum Dis Clin North Am. 2008;34(1):57-71. doi:10.1016/j.rdc.2007.12.004.
- Kill A, Riemekasten G. Functional autoantibodies in systemic sclerosis pathogenesis. Curr Rheumatol Rep. 2015;17(5):34. doi:10.1007/s11926-015-0505-4.
- Anderson S, King A J, Brenner B M. Hyperlipidemia and Glomerular Sclerosis: An Alternative Viewpoint. The American Journal of Medicine 1989; 87; 5-34N – 5-38N.
- Neumann H A M, Van den Broek M J T B. Increased collagen IV layer in the basal membrane area of the capillaries in severe chronic venous insufficiency. VASA 1991; 20; 26-29.
- Vicaut E. Opposite effects of red blood cell aggregation on resistance to blood flow. The Journal of Cardiovascular Surgery. 1995 Aug; 36(4): 361-8.
- Zaragoza C, Márquez S, Saura M. Endothelial mechanosensors of shear stress as regulators of atherogenesis. Curr Opin Lipidol. 2012;23(5):446-452. doi:10.1097/MOL.0b013e328357e837.
- Vekasi J, Marton ZS, Kesmarky G, Cser A, Russai R, Horvath B. Hemorheological alterations in patients with diabetic retinopathy. Clin Hemorheol Microcirc. 2001;24(1):59-64.
- Baskurt OK, Meiselman HJ. Erythrocyte aggregation: Basic aspects and clinical importance. Clin Hemorheol Microcirc. 2013;53(1-2):23-37. doi:10.3233/CH-2012-1573.
- Barcellini W. New Insights in the Pathogenesis of Autoimmune Hemolytic Anemia. Transfus Med Hemother. 2015;42(5):287-293. doi:10.1159/000439002.
- Franco RS, Puchulu-Campanella ME, Barber LA, et al. Changes in the properties of normal human red blood cells during in vivo aging. Am J Hematol. 2013;88(1):44-51. doi:10.1002/ajh.23344.
- Weber H, Schmid-Schonbein H, Lemmens H A J. Plasmapheresis as a Treatment of Raynaud’s Attacks: Microrheological Differential Diagnosis and Evaluation of Efficacy. Clinical Hemorheology 1985; 5; 85-97.
- Piccini JP, Nilsson K. The Osler Medical Handbook: Mobile Medicine Series, 2e. Johns Hopkins Hospital. 2006 Aug;36(4):361-8.
- Gudmundsson M, Bjelle A. Viscosity of plasma and blood in rheumatoid arthritis. Br J Rheumatol. 1993;32(9):774-779.
- Rosenson RS, Shott S, Katz R. Elevated blood viscosity in systemic lupus erythematosus. Semin Arthritis Rheum. 2001;31(1):52-57. doi:10.1053/sarh.2001.24876.
- Dwosh IL, Giles AR, Ford PM, Pater JL, Anastassiades TP, Group and the QUPS. Plasmapheresis Therapy in Rheumatoid Arthritis. N Engl J Med. 1983;308(19):1124-1129. doi:10.1056/NEJM198305123081903.
- Wei N, Klippel JH, Huston DP, et al. Randomised trial of plasma exchange in mild systemic lupus erythematosus. Lancet (London, England). 1983;1(8314-5):17-22.
- von Rhede van der Kloot E J H, Jacobs M J H M, Weber H, Lemmens H A J. Plasma Filtration in Patients with Raynaud’s Phenomenon. Clinical Hemorheology 1985; 5; 79-84.
- Jacobs M J H M, Breslau P J, Slaaf D W, Reneman R S, Lemmens J A J. Nomenclature of Raynaud’s phenomenon: A capillary microscopic and hemorheologic study. Surgery 1987; 101; 136-145.
- Pais E, Alexy T, Holsworth RE Jr, Meiselman HJ. Effects of nattokinase, a pro-fibrinolytic enzyme, on red blood cell aggregation and whole blood viscosity. Clinical Hemorheology and Microcirculation 2006: 35(1-2); 139-42.
- Toth K, Wenby RB, Meiselman HJ. Inhibition of polymer-induced red blood cell aggregation by poloxamer 188. Biorheology. 2000;37(4):301-312.
- Xian-Qiang Mi, Dr. Ji-Yao Chen, Zi-Jun Liang, and Lu-Wei Zhou. Photomedicine and Laser Surgery. December 2004, 22(6): 477-482. https://doi.org/10.1089/pho.2004.22.477
- Patten E, Berkman EM. Therapeutic Plasmapheresis and Plasma Exchange. CRC Crit Rev Clin Lab Sci. 1986;23(2):147-175. doi:10.3109/10408368609165798.
- O’Reilly MJ, Talpos G, Roberts VC, White JM, Cotton LT. Controlled trial of plasma exchange in treatment of Raynaud’s syndrome. British Medical Journal. 1979 Apr 28;1(6171):1113-5.
- Romano E, Manetti M, Guiducci S, Ceccarelli C, Allanore Y, Matucci-Cerinic M. The genetics of systemic sclerosis: an update. Clin Exp Rheumatol. 29(2 Suppl 65):S75-86.
- Tripette J, Alexy T, Hardy-Dessources M-D, et al. Red blood cell aggregation, aggregate strength and oxygen transport potential of blood are abnormal in both homozygous sickle cell anemia and sickle-hemoglobin C disease. Haematologica. 2009;94(8):1060-1065. doi:10.3324/haematol.2008.005371.
- Hebbel RP, Osarogiagbon R, Kaul D. The Endothelial Biology of Sickle Cell Disease: Inflammation and a Chronic Vasculopathy. Microcirculation. 2004;11(2):129-151. doi:10.1080/10739680490278402.
- McCune MA, Winkelmann RK, Osmundson PJ, Pineda AA. Plasma exchange: a controlled study of the effect in patients with Raynaud’s phenomenon and scleroderma. Journal of Clinical Apheresis. 1983;1(4):206-14.
- Di Spaltro, FX, Cottrill, C, Cahill, C, Degnan, E, Mulford, GJ, Scarborough, D, Franks, AJ, Klainer, AS, Bisaccia, E. Extracorporeal photochemotherapy in progressive systemic sclerosis. Int J Dermatol, 1993 32(6), 417-421.
- Krasagakis K, Dippel E, Ramaker J, Owsianowski M, Orfanos CE. Management of severe scleroderma with long-term extracorporeal photopheresis. Dermatology. 1998. 309-15.
- Rook AH, Freundlich B, Jegasothy BV, Perez MI, Barr WG, Jimenez SA, et al. Treatment of systemic sclerosis with extracorporeal photochemotherapy. Results of a multicenter trial. Arch Dermatol. 1992 Mar; 128(3): 337-46.
- Wollina U, Oelzner S, Looks A, Hipler UC, Knoll B, Lange D, et al. [Progressive systemic sclerosis – treatment results of extracorporeal photopheresis]. Hautarzt. 1999. 637-42.
- Knobler RM, French LE, Kim Y, Bisaccia E, Graninger W, Nahavandi H, Strobl FJ, Keystone E, Mehlmauer M, Rook AH, Braverman I. A randomized, double-blind, placebo-controlled trial of photopheresis in systemic sclerosis. J Am Acad Dermatol. Volume 54. 2006. 793-799.
- Muangchan C, Harding S, Khimdas S, Bonner A, Baron M, Pope J. Association of C-reactive protein with high disease activity in systemic sclerosis: results from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken). 2012;64(9):1405-1414. doi:10.1002/acr.21716.
- Baskurt, O; Björn, N; Meiselman H. Red Blood Cell Aggregation. CRC Press; 2011.
- Shand L, Lunt M, Nihtyanova S, et al. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: Application of a latent linear trajectory model. Arthritis Rheum. 2007;56(7):2422-2431. doi:10.1002/art.22721.
- Meier S, Mikuls TR. Positive Predictive Value of Anti-Centromere and Anti-Scl-70 Antibody Multiplex Assays in a Rheumatology Practice Setting. Arthritis Rheum. 2011;63(Suppl 10):694.
