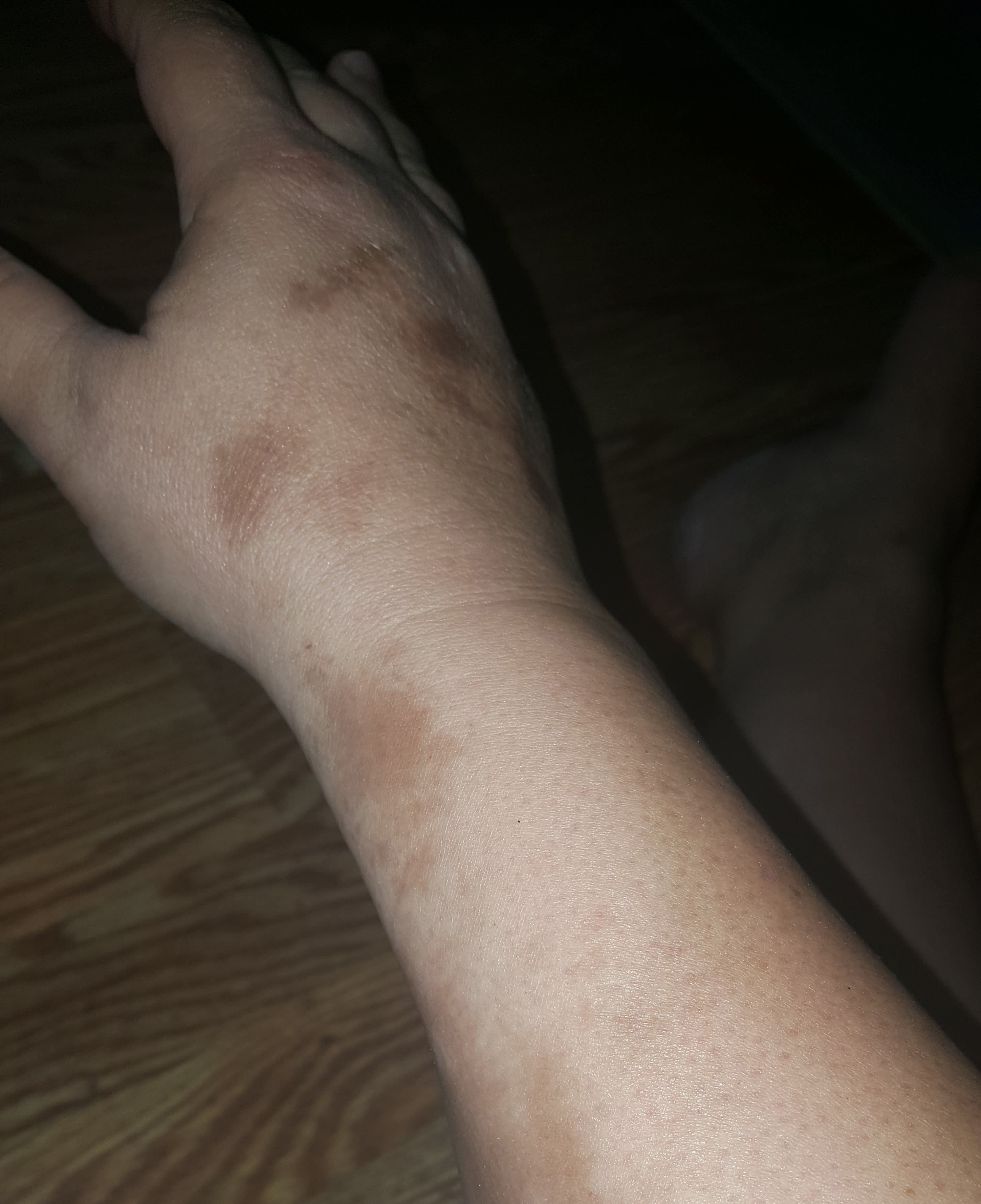
Localized Scleroderma and Scleroderma-Like Disorders
Morphea, or localized scleroderma, can affect all ages and is more common in women. It typically presents as patches of yellowish or ivory-colored rigid, dry skin. These are followed by the appearance of firm, hard, oval-shaped plaques with ivory centers that are encircled by a violet ring. These spots generally appear on the trunk, face, and/or extremities. Many patients with localized morphea improve without treatment. Generalized morphea is more rare and serious and involves the skin but not the internal organs.
Linear scleroderma appears as a band-like thickening of skin on the arms or legs. This type of scleroderma is most likely to be on one side of the body but may be on both sides. Linear scleroderma generally appears before age 20. When it occurs in young children, it may result in the failure of one limb (e.g., an arm or leg) to grow as rapidly as its counterpart.
Diffuse fasciitis with eosinophilia (DFE, also called eosinophilic fasciitis or Shulman’s syndrome) is a rare condition that mimics scleroderma with swelling, stiffness, and decreased flexibility of the limbs associated with skin thickening. Although the symptoms can be widespread and involve the trunk and limbs, in contrast to scleroderma, the fingers, hands, and face are usually not affected. In addition, there is no occurrence of Raynaud’s or GI involvement.
Eosinophilia-myalgia syndrome (EMS) is a rare condition that was first described after 3 patients in New Mexico were found to have an illness with significant myalgia (muscle pain) and an increase in the number of eosinophils (a type of white blood cell). All three patients had taken supplements containing L-tryptophan, which may have been contaminated. All told, about 1500 people were affected. A similar outbreak occurred in Spain in 1981 and affected almost 20,000 people. As it may have been the result of consuming contaminated rapeseed oil, it was known as toxic oil syndrome (TOS). About 60% of the patients developed skin thickening that look like skin changes typical for scleroderma patients, although the affected areas were different than what is normally seen with scleroderma and there is no associated Raynaud’s phenomenon.
Scleroderma-like skin changes have also been associated with insulin-dependent diabetes, carcinoid syndrome, myeloma, scleromyxedema, chronic graft-versus-host disease, porphyria cutanea tarda, Werner’s syndrome, progeria, phenylketonuria, bleomycin exposure, local lipodystrophies, nephrogenic fibrosing dermopathy, and POEMS syndrome.
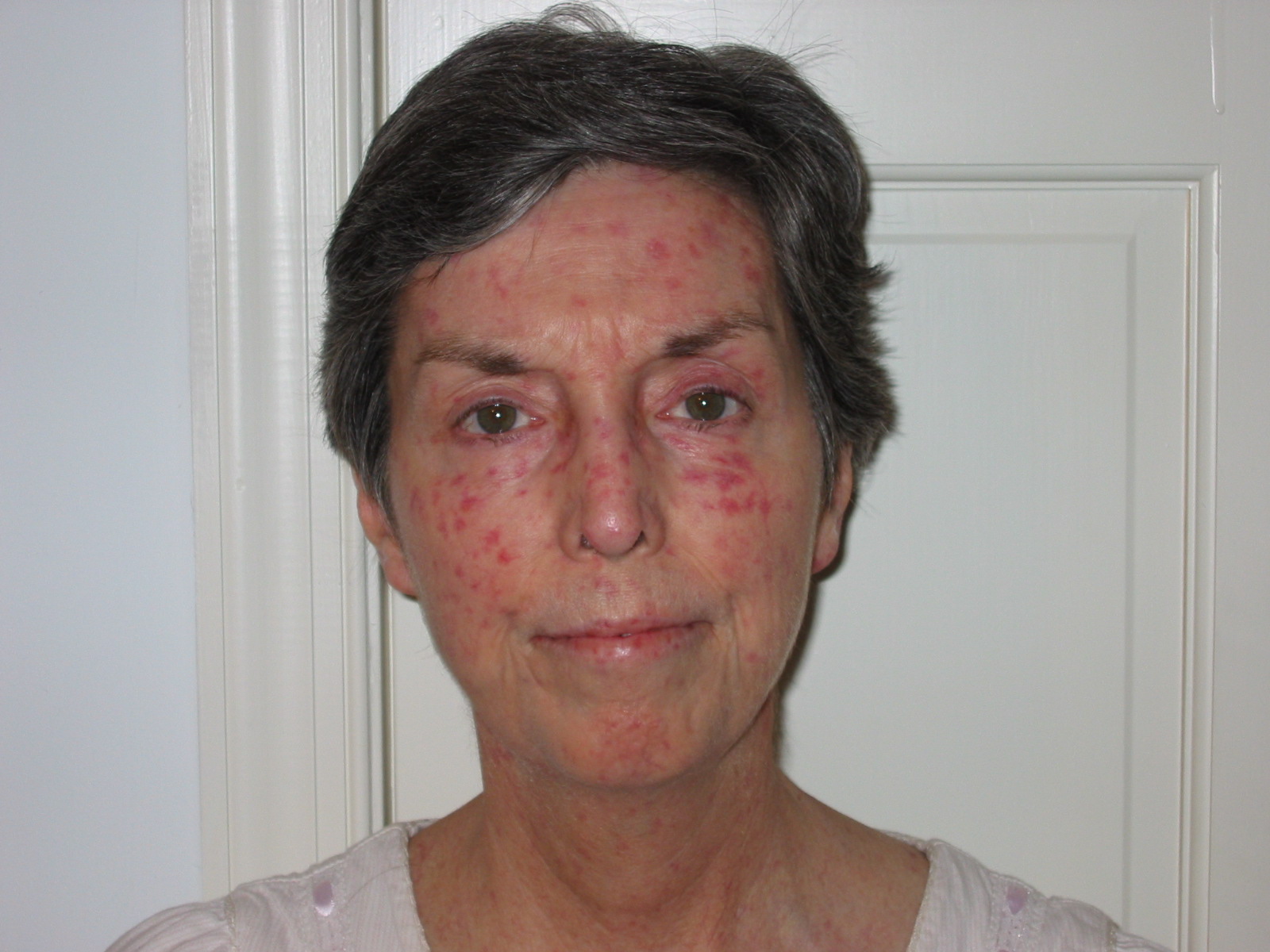
Systemic Scleroderma
Systemic scleroderma diagnosis is often a challenging and lengthy process. It is not uncommon for a person who ultimately is diagnosed with one of the forms of systemic scleroderma to be initially misdiagnosed with many different disorders. Part of the reason for this is that some early scleroderma symptoms are non-specific, and unless the physician suspects scleroderma, s/he may not order the appropriate tests to diagnose the condition.
Scleroderma and ANA (Anti-nuclear Antibody) Testing
In almost all cases of systemic scleroderma, the patient will have a positive anti-nuclear antibody (ANA) test result. However, even this test can be problematic. There are now several different ways of testing for ANA. The long-term “gold standard” is a method called indirect immunofluorescence (commonly abbreviated as IFA or IIF). This has very high reliability and is the best way to test for the presence of anti-nuclear antibodies. However, it is a complex and time consuming test that depends on highly trained laboratory personnel. Recently, many commercial laboratories and some larger hospital laboratories have switched their routine ANA testing to solid phase immunoassays (ELISA or EIA) or a related technique known as a Multiplex platform. These new techniques can handle high testing volumes since they are not labor intensive like IFA testing and are, therefore, less expensive than IFA. However, these new methods of testing can only detect a limited subset of the specific antibodies that are targeted by the tests (typically 8-10) in contrast to IFA that can detect 100 to 150 different possible antibodies. As a result, these alternate testing methods are more likely to miss relevant autoantibodies yielding false negative ANA results. For example, a recent study (Shanmugam et al. 2011) reported that up to 43% of scleroderma patients with positive ANA results by IFA yielded negative ANA results using the Multiplex method. This can have major impact on scleroderma diagnosis. If the results of an initial ANA screening come back negative to the doctor who ordered the ANA test without knowing this data, this can be the start of (in some cases) years of diagnostic limbo for patients. By the time they are finally retested for ANA by the more comprehensive IFA method, their symptoms will have progressed and may be more difficult to treat.
Learn more about Scleroderma Antibodies and Clinical Relevance.
New Formal Diagnostic Criteria for Systemic Scleroderma
In late 2013, the American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) approved a new set of diagnostic criteria for systemic scleroderma, replacing the older 1980 diagnostic criteria (van den Hoogen et al. 2013). These new standards will improve clinical diagnosis of systemic scleroderma, but it is very important to understand that the reason for developing these new diagnostic standards was “to develop a set of criteria that would enable identification of individuals with SSc for inclusion in clinical studies,” not for normal diagnosis of patients in a clinical setting. The authors of the special report that formally introduces the new criteria note that many symptoms that are used for clinical diagnosis are not included in these formal research criteria, including common symptoms such as tendon friction rubs, calcinosis, difficulty swallowing, as well as less common but more serious complications such as renal crisis.
Note: Table 1a is a simplified version of the new classification criteria:
Table 1a: 2013 ACR/EULAR Classification Criteria for Systemic Scleroderma
Item |
Sub-Item(s) |
Weight |
| Skin thickening of the fingers of both hands that extends at least up to the joint at the base of the fingers (third joint on fingers, second joint on thumb) (sufficient criterion) |
9 |
|
| Skin thickening of the fingers (only count the higher score) | Puffy fingers |
2 |
| Thickening of the fingers up to the second finger joint |
4 |
|
| Fingertip lesions (only count the higher score) | Digital tip ulcers |
2 |
| Fingertip pitting scars |
3 |
|
| Telangiectasia |
2 |
|
| Abnormal nailfold capillaries |
2 |
|
| Pulmonary arterial hypertension and/or interstitial lung disease (maximum score 2) | Pulmonary arterial hypertension |
2 |
| Interstitial lung disease |
2 |
|
| Raynaud’s phenomenon (can be self-reported) |
3 |
|
| Scleroderma-related autoantibodies (maximum score 3) | Anti-centromereAnti-Scl-70 (Anti–topoisomerase I)Anti–RNA polymerase III |
3 |
The total score is determined by adding the maximum weight (score) in each category. Patients with a total score of 9 or greater are classified as having definite systemic scleroderma. For example, a patient with definite skin thickening on both hands all the way to the base of the fingers receives a score of 9 just for that single symptom and is automatically classified as having definite systemic scleroderma. For the other categories, you receive points based on the highest scoring symptom in that category. To illustrate, a patient that has Raynaud’s (3), fingertip lesions with pitting scars (3), anti-centromere antibodies (3), and abnormal nailfold capillaries (2) would receive a total weighted score of 11 and would also be diagnosed with systemic scleroderma. Note that within a general category, e.g., “Skin thickening of the fingers”, you would “earn” 4 points for skin thickening up to the second finger joint OR 2 points if you just had puffy fingers, but not 6 points for both.
There is no question that these new diagnostic criteria will be helpful to clinicians as well as researchers, but there are a number of issues that will arise in clinical diagnosis because of the way these criteria were developed. For example, you will note that there is nothing in these criteria that includes any GI involvement, which is very common with all forms of systemic scleroderma. There is also no mention of renal (kidney) problems, which are rare but a strong clinical complication that occurs with some forms of systemic scleroderma.
These were excluded from these research criteria for different reasons. In the case of GI symptoms such as GERD (reflux), from a research classification perspective they are not specific enough to just systemic scleroderma to be useful in patient classification, since they can occur with many other different diseases, e.g., lupus. On the other hand, while renal crisis associated with some of the other symptoms is very specific to systemic scleroderma, it is actually so rare that it didn’t reach the level of significance in doing the classification research, so there was no benefit to including it in the classification criteria.
It is also very noteworthy that the “Scleroderma-related autoantibodies” category adds anti-RNA polymerase III to the standard anti-centromere and anti-Scl-70 antibodies that have been associated with systemic scleroderma for many years. As mentioned above, the anti-RNA polymerase III antibody is associated with one of the diffuse variants of scleroderma and has a different typical clinical symptom profile than diffuse patients with the anti-Scl70 antibody (see Table 2). Also, the paper discussed additional antibodies indicating that they are likely to be added to the table in the future as more research is done to allow better understanding of the clinical significance of these less common antibodies. However, it is worth noting that the new criteria only results in a diagnosis of systemic scleroderma, but does not directly indicate which form of scleroderma, even at the general level of limited or diffuse, in spite of directly including three specific antibodies in the table.
Scleroderma diagnosis will remain a clinical challenge in many cases, notwithstanding the new diagnostic criteria. For example, clinicians still need to consider clinical symptoms that support a diagnosis of systemic scleroderma that are not included in the new 2013 ACR criteria, e.g., GI symptoms such as GERD (reflux), difficulty swallowing, muscle pain, etc. An additional challenge for physicians is the switch to a new ICD10 diagnostic coding system that occurred in October 2015 (see note below). This will require more specific diagnosis than is currently required.
The reality is that in most cases, when patients start developing symptoms such as Raynaud’s, heartburn, puffy fingers, muscle pain and weakness, their first visit will be to their primary care physician, who is likely to be an internist, family medicine doctor, or a nurse practitioner. In most cases, these physicians will have rarely, if ever, encountered a patient with scleroderma and may not have read anything about the disease since they were in medical school 20 years earlier! Because of the rarity of systemic scleroderma, many primary care physicians may not initially think of autoimmune diseases. However, once the patient or physician starts to consider a potential autoimmune disease as the cause of the patient’s symptoms, it is almost always the best course of action to bring a rheumatologist into the diagnostic loop since s/he will be trained in diagnosing and treating autoimmune diseases. It is still important to realize that, especially in a small community, most rheumatologists may have never seen a patient with scleroderma, but at least they are much more likely to have the training needed to correctly diagnosis scleroderma and work with the patient to determine the best treatment options for his/her particular situation.
At a final level, there are now a number of clinics (at least in the US) that specialize in scleroderma diagnosis and treatment. The Scleroderma Foundation (www.scleroderma.org) is a good resource for locating scleroderma specialty clinics. The list of research and treatment centers is located under the tab “Healthcare Professional”.
“Sine” Scleroderma
“Sine scleroderma” is a term that is used to describe cases of systemic scleroderma where there is internal organ involvement that is characteristic of scleroderma, but with no skin thickening. It is described as a rare variant of scleroderma in several online articles about scleroderma, but the term almost never appears in scleroderma research literature. In some of the few studies that have looked at the characteristics of patients with sine scleroderma, it is mostly associated with limited forms of scleroderma rather than diffuse forms, and is generally considered to have a good prognosis. While there can be skin abnormalities, such as telangiectasias and abnormal nailfold capillaries, the skin thickening which is the hallmark symptom of all forms of scleroderma is not present in these patients.
A number of researchers have commented that sine scleroderma is really nothing more than a symptom variant of either the limited or diffuse forms of scleroderma, in the same way that lung involvement is a symptom variant in both forms of the disease (Diab et al. 2014). Classically, with limited scleroderma it is very common for patients to have a symptom progression that begins with Raynaud’s, is followed by “puffiness” of the fingers, especially in the morning, abnormal nailfold capillaries, and GI symptoms (primarily reflux) for many years before actual skin thickening is noted. Internal organ damage is typically later with limited scleroderma as well, but can sometimes occur early in the disease process, creating the potential for the “sine” condition. In most cases, skin changes do eventually occur even with limited scleroderma, but in other cases they may never reach diagnostic significance during the overall course of the disease.
With the more rapidly progressing diffuse forms of scleroderma, skin changes typically occur earlier and progress more rapidly. However, internal organ damage typically appears much earlier than with limited scleroderma, sometimes before even Raynaud’s symptoms or skin changes are evident, so the sine state is possible here as well, although less often than with limited scleroderma.
From a diagnostic standpoint, having a cluster of symptoms that can be associated with systemic scleroderma but without visible skin changes can be a major problem. When skin thickening is evident, especially when accompanied by Raynaud’s and reflux, many primary care physicians will have the training to recognize that this is likely to be an autoimmune disease and either order the appropriate diagnostic tests to try to determine this, or alternatively, refer the patient to a rheumatologist. However, if someone comes in complaining of muscle and joint pain, reflux, and shortness of breath, for example, most physicians will not automatically think about an autoimmune disease, much less scleroderma. Even if the patient also has mild Raynaud’s, they may not think of this as being anything more than that they have cold hands and, thus, may not even mention it to their physician. This makes diagnosis very challenging, and, unfortunately, especially in cases with no visible skin involvement, getting a proper diagnosis can sometimes take literally years and be very frustrating for the patient (as well as their physicians).
A Note About Scleroderma Diagnostic Coding
Until October 2015, hospitals and physician used a diagnostic coding system called ICD9 for billing. ICD9 had only one code for systemic scleroderma – 710.1. All variants of systemic scleroderma, including Progressive Systemic Sclerosis (old name for diffuse scleroderma) and CREST syndrome (old name for limited scleroderma) were lumped together under this single billing code. From a practical standpoint, it meant that Medicare and private insurance companies did not distinguish between the two general categories of systemic scleroderma when deciding what medications and treatment options would be covered.
On October 1, 2015, ICD9 was replaced with ICD10. Under ICD10, all variants of systemic scleroderma are grouped under a general billing code of M34. However, for the first time, there are specific scleroderma subcategories under the general M34 classification. Even though the old names are still being used, there are now separate diagnostic categories for diffuse scleroderma (M34.0) and limited scleroderma (M34.1). There is also a specific code for systemic scleroderma caused by known exposure to drugs or toxic chemicals (M34.2).
However, there are also two other new subcategories that are unfortunately going to create a lot of confusion for physicians. Table 1b shows the new ICD10 classification codes:
Table 1b: ICD10 Coding for Systemic Scleroderma
Code |
Description |
Notes |
| M34.0 | Progressive systemic sclerosis | Diffuse scleroderma |
| M34.1 | CR(E)ST syndrome | Limited scleroderma |
| M34.2 | Systemic sclerosis induced by drug or chemicals | |
| M34.8 | Other forms of systemic sclerosis: | |
| – M34.81 | – Systemic sclerosis with lung involvement | |
| – M34.82 | – Systemic sclerosis with myopathy | Muscle pain and weakness |
| – M34.83 | – Systemic sclerosis with polyneuropathy | Nerve damage and weakness |
| – M34.89 | – Other systemic sclerosis | |
| M34.9 | System sclerosis, unspecified |
Where the confusion is likely to occur is with the M34.8 subcategory codes, e.g., M34.81 for scleroderma with lung involvement. Since lung involvement is possible with both limited and diffuse scleroderma, it is not clear how clinicians will decide on a diagnostic code for patients that are known to be diffuse or limited, based on antibody profile and symptoms, but also have lung involvement. It is also not clear how physicians will distinguish between M34.89 and M34.9 coding, since there are currently no clear guidelines for these subcategories. Presumably the ICD10 codes will become better defined over time. In the short term, other than the need to decide on how to translate the general ICD9 code for scleroderma into the more specific ICD10 codes, there will probably be little impact on patients because of the forthcoming diagnostic coding change. However, at some point in the future, insurance companies could potentially decide which kinds of treatments will be covered based on the specific scleroderma type with which the patient is formally diagnosed with.
